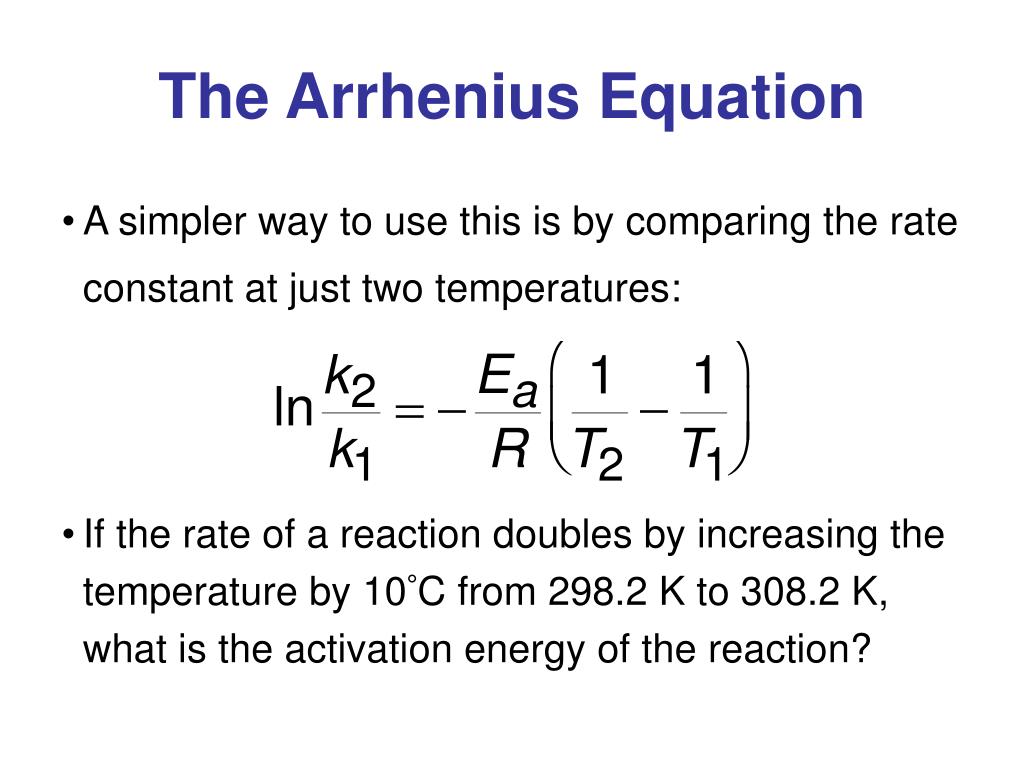
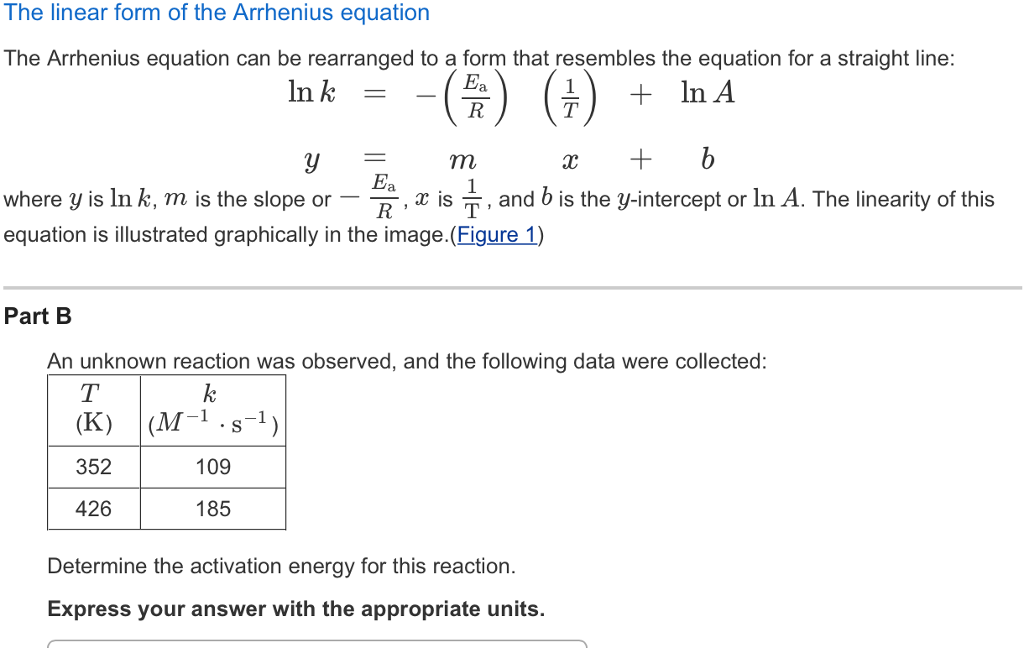
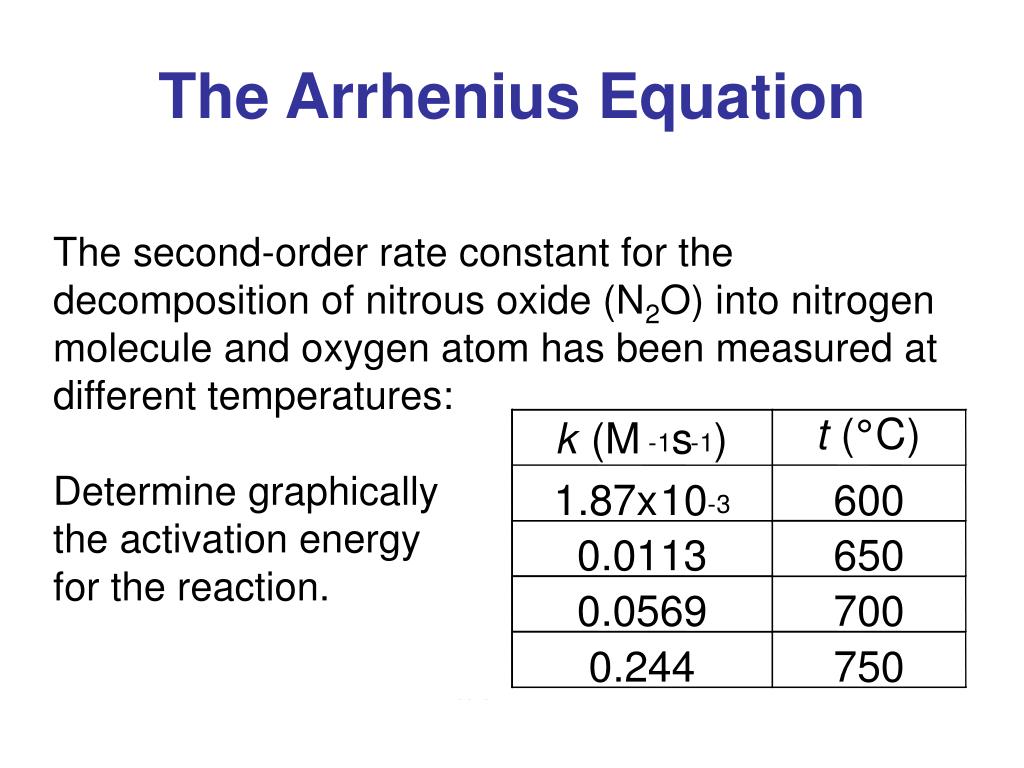
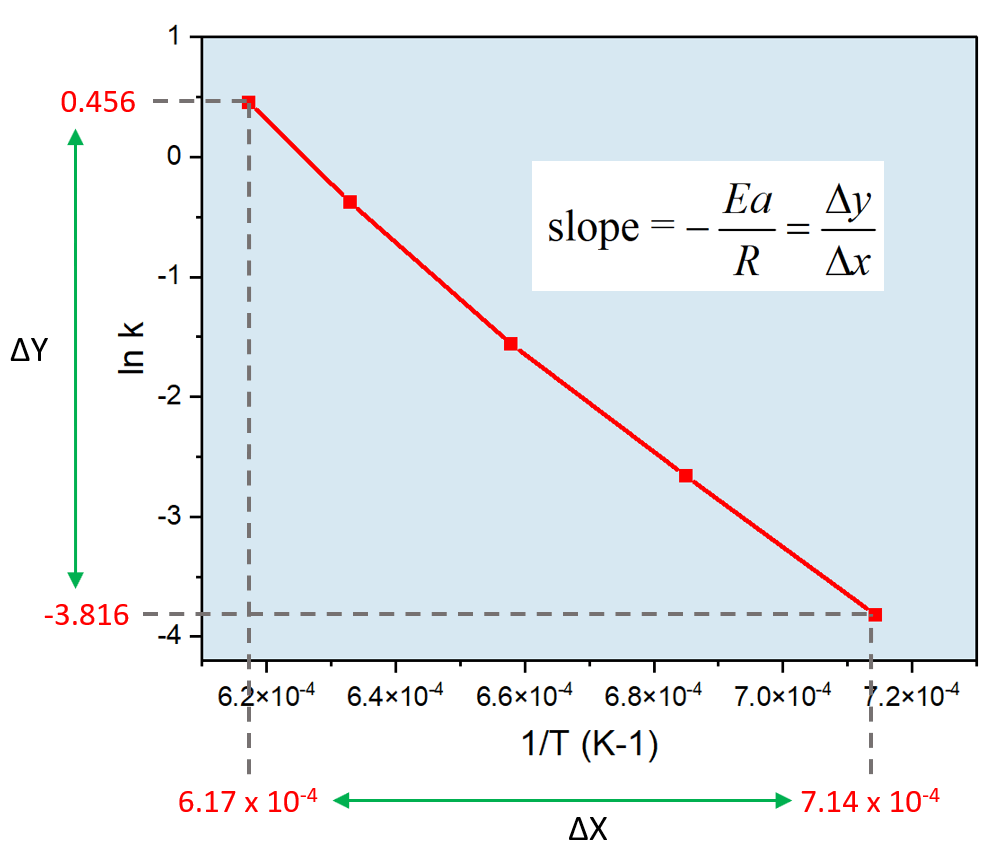
Arrhenius Equation Linear Form
Arrhenius Equation Linear Form - The arrhenius equation is applicable to various reaction systems. Web the arrhenius equation k a e e a. The expression that gives the relationship between rate constant and temperature is known as the arrhenius. Web it means that temperature affects the rate of reaction. The linear form of the arrhenius equation the arrhenius equation can be rearranged to a form that resembles the equation for a straight line: This means a plot of ln. This has the form y=mx. When linearizing the equation into the form of y=mx+b, we get. Now we want to solve for. R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a.
Lowering the activation energy of a reaction by a catalyst. When linearizing the equation into the form of y=mx+b, we get. Web the arrhenius equation k a e e a. Ln(kr)= −ea r × 1 t +ln(a) l n ( k r) = − e a r × 1 t + l n ( a) where the slope of the. If you need to use this equation, just find the ln button on your calculator. This means a plot of ln. The concept of activation energy explains the exponential nature of the relationship, and in one way or another, it is present in all kinetic theories. Web it means that temperature affects the rate of reaction. Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. Web the arrhenius equation can be derived from experimental data.
Lowering the activation energy of a reaction by a catalyst. This has the form y=mx. Ln(kr)= −ea r × 1 t +ln(a) l n ( k r) = − e a r × 1 t + l n ( a) where the slope of the. Now we want to solve for. Web ln is a form of logarithm. It is easy to predict the value for the rate. Web the arrhenius equation k a e e a. R.t take the natural log ln(k ) ln a e e a. The linear form of the arrhenius equation the arrhenius equation can be rearranged to a form that resembles the equation for a straight line: When linearizing the equation into the form of y=mx+b, we get.
PPT The Arrhenius Equation PowerPoint Presentation, free download
The linear form of the arrhenius equation the arrhenius equation can be rearranged to a form that resembles the equation for a straight line: At an absolute temperature t, the fraction of molecules that have a kinetic energy greater than ea can be calculated from statistical mechanics. Web the arrhenius equation can be derived from experimental data. It is easy.
6 Plotting of the linear form of the Arrhenius equation provides a
Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. At an absolute temperature t, the fraction of molecules that have a kinetic energy greater than ea can be calculated from statistical mechanics. R.t expand expression.
Solved The Linear Form Of The Arrhenius Equation The Arrh...
Once activation energy for a reaction is known; The arrhenius equation is applicable to various reaction systems. Web the arrhenius equation can be expressed in a more applicable form by taking the natural logarithm of both sides which gives a form of a linear equation: Arrhenius argued that for reactants to transform into products, they must first acquire a minimum.
16.2 The Arrhenius equation (HL) YouTube
Web the arrhenius equation k a e e a. If you need to use this equation, just find the ln button on your calculator. It is easy to predict the value for the rate. The concept of activation energy explains the exponential nature of the relationship, and in one way or another, it is present in all kinetic theories. Don't.
12.6 Arrhenius Equation YouTube
R.t take the natural log ln(k ) ln a e e a. Ln(kr)= −ea r × 1 t +ln(a) l n ( k r) = − e a r × 1 t + l n ( a) where the slope of the. Lowering the activation energy of a reaction by a catalyst. Arrhenius argued that for reactants to transform into.
The other forms of the Arrhenius Equation YouTube
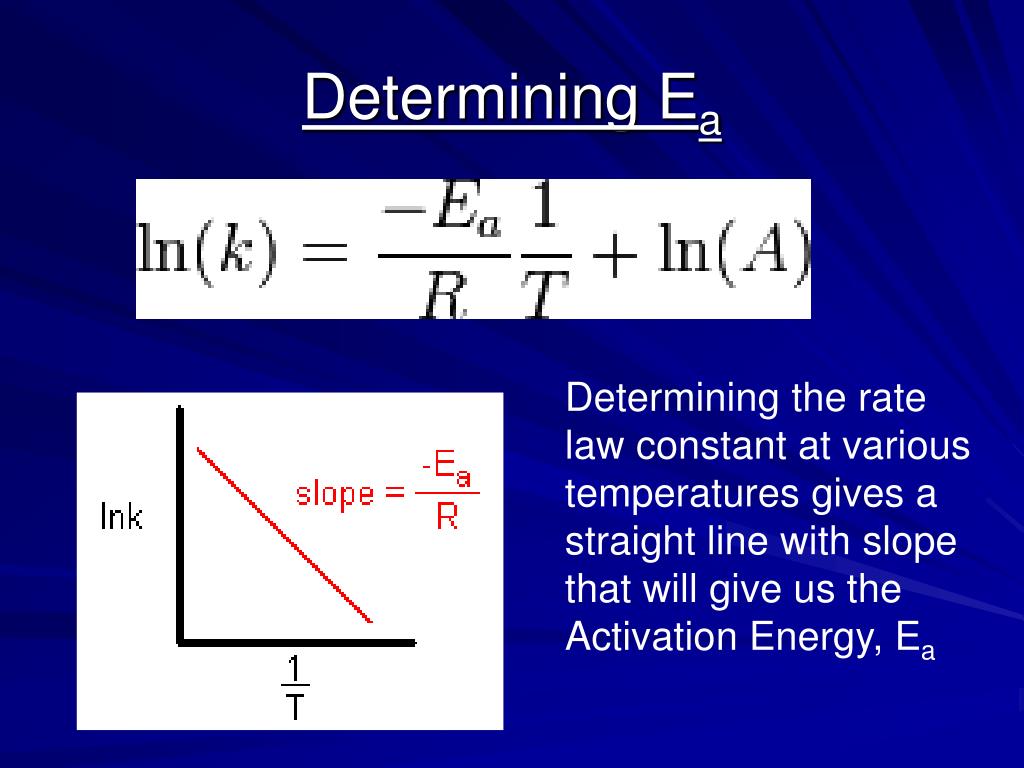
When linearizing the equation into the form of y=mx+b, we get. A is a constant for a. Web ln is a form of logarithm. Web the arrhenius equation is in linear form if we graph ln(kr) l n ( k r) as y and 1 t 1 t as x: Web the arrhenius equation k a e e a.
PPT The Arrhenius Equation PowerPoint Presentation, free download
This means a plot of ln. Ln(kr)= −ea r × 1 t +ln(a) l n ( k r) = − e a r × 1 t + l n ( a) where the slope of the. The expression that gives the relationship between rate constant and temperature is known as the arrhenius. This has the form y=mx. When linearizing the.
Arrhenius equation chemistry steps (2023)
R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a. Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. Ln(kr)= −ea r × 1 t +ln(a) l n (.
The Arrhenius Equation (A Level Chemistry) Teaching Resources
The concept of activation energy explains the exponential nature of the relationship, and in one way or another, it is present in all kinetic theories. Web ln is a form of logarithm. When linearizing the equation into the form of y=mx+b, we get. If you need to use this equation, just find the ln button on your calculator. It is.
PPT The Arrhenius Equation PowerPoint Presentation, free download
R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a. Linear form \[\ln k = \dfrac{. The expression that gives the relationship between rate constant and temperature is known as the arrhenius. Now we want to solve for. Ln(kr)= −ea r × 1 t +ln(a) l n ( k r) = − e a.
The Concept Of Activation Energy Explains The Exponential Nature Of The Relationship, And In One Way Or Another, It Is Present In All Kinetic Theories.
Web the arrhenius equation k a e e a. The expression that gives the relationship between rate constant and temperature is known as the arrhenius. Now we want to solve for. This means a plot of ln.
R.t Expand Expression (Multiplied Inside Log So Add) Ln(K ) Ln(A ) Ln E E A.
Don't worry about what it means. When linearizing the equation into the form of y=mx+b, we get. Linear form \[\ln k = \dfrac{. The arrhenius equation is applicable to various reaction systems.
R.t Take The Natural Log Ln(K ) Ln A E E A.
Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. A is a constant for a. The linear form of the arrhenius equation the arrhenius equation can be rearranged to a form that resembles the equation for a straight line: Web it means that temperature affects the rate of reaction.
Web The Arrhenius Equation Can Be Derived From Experimental Data.
Lowering the activation energy of a reaction by a catalyst. Once activation energy for a reaction is known; Ln(kr)= −ea r × 1 t +ln(a) l n ( k r) = − e a r × 1 t + l n ( a) where the slope of the. This has the form y=mx.