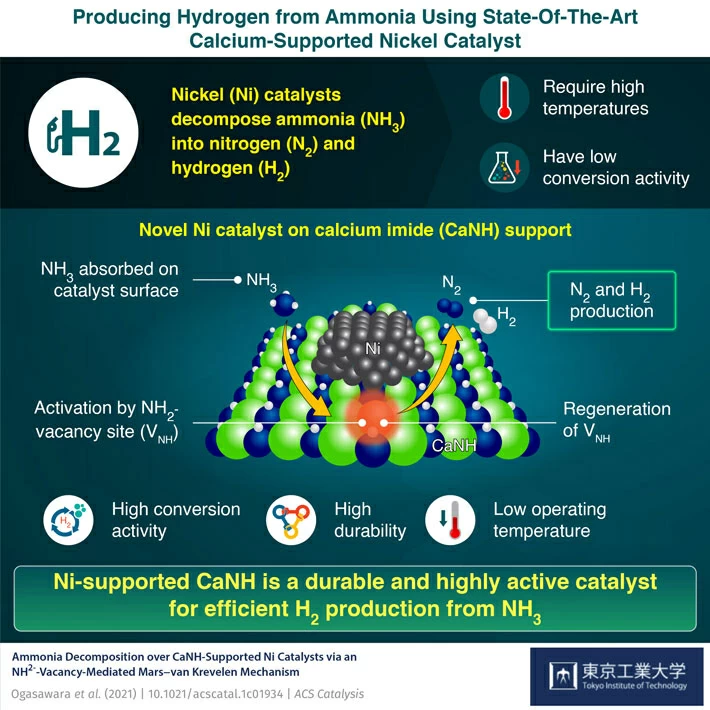
Can Ammonia Form Hydrogen Bonds
Can Ammonia Form Hydrogen Bonds - The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more. In a group of ammonia molecules, there aren't enough. Of course it can interact with positively polarized hydrogens in an electrostatic way. The lone pair of electrons present in nitrogen is equal to one. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. In the gaseous state at high temperature, the ammonia molecules have sufficient kinetic energy to overcome the attractions to other ammonia molecules. Web in the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. Liquid ammonia is constituted of ammonia molecules. Hydrogen bonding is the intermolecular forces acting between ammonia molecules.
When the hydrogen bonds between water molecules are broken, they can be replaced by equivalent bonds. Web ammonia can form four hydrogen bonds per molecule. Web answer (1 of 3): Web in the ammonia molecule group, the lone pair of electrons is not enough for forming the hydrogen bond. Spectroscopic characterizations of the stereochemistry. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. Of course it can interact with positively polarized hydrogens in an electrostatic way. Theoretical studies of the reaction. Due to the electronegativity difference. The lone pair on nitrogen can accept one hydrogen to form a hydrogen bond, and the three hydrogen.
Web answer (1 of 3): The lone pair on nitrogen can accept one hydrogen to form a hydrogen bond, and the three hydrogen. Web ammonia is an important molecule due to its wide use in the fertiliser industry. 1 ammonia (nh 3) forms a weakly hydrogen. Web in ammonia (nh 3 ), the number of hydrogen bonds formed is limited since in each nitrogen there is only one lone pair of electrons which is shared with one hydrogen. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more. When the hydrogen bonds between water molecules are broken, they can be replaced by equivalent bonds. Web in the ammonia molecule group, the lone pair of electrons is not enough for forming the hydrogen bond. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds.
Form I333 Anhydrous Ammonia Additive Credit printable pdf download
Web in the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair. Web ammonia is an important molecule due to its wide use in the fertiliser industry. 8.9k views 1 year ago. 1 ammonia (nh 3) forms a weakly hydrogen. Web paradoxically ammonia is a better carrier of.
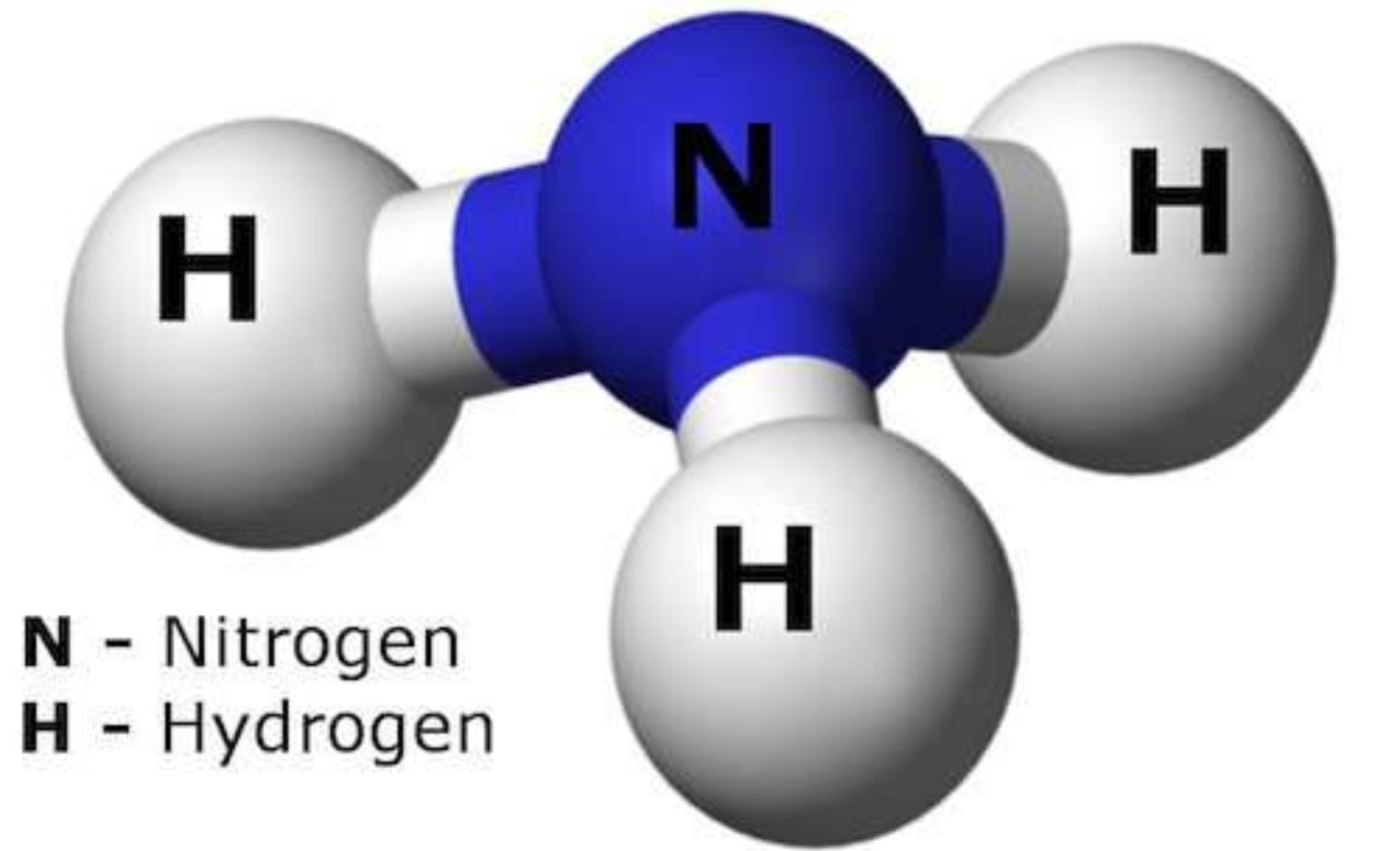
Ammonia Molecule Structure built with Atoms & Bonds from
The lone pair on nitrogen can accept one hydrogen to form a hydrogen bond, and the three hydrogen. In a group of ammonia molecules, there aren't enough. Web an ammonia molecule can donate and accept up to three hydrogen bonds. Web ammonium ion still has nitrogen bearing a partial negative charge. Ammonia clusters are constituted of ammonia molecules linked by.
What is Ammonia? nh3 fuels
Web ammonia can form four hydrogen bonds per molecule. Web these three materials have four fundamental elements, o, n, c, and h, that are the building blocks of amino acids. Spectroscopic characterizations of the stereochemistry. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. In the gaseous state at high temperature, the.
Can Ammonia aka NH3 Be a Fuel?
Web as expected, nh(3) is observed to be a nearly universal proton acceptor, accepting hydrogen bonds fr. Hydrogen bonding is the intermolecular forces acting between ammonia molecules. In the gaseous state at high temperature, the ammonia molecules have sufficient kinetic energy to overcome the attractions to other ammonia molecules. 1 ammonia (nh 3) forms a weakly hydrogen. Web actually, an.
chemistry Intermolecular Hydrogen Bonding
Web an ammonia molecule can donate and accept up to three hydrogen bonds. The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more. When the hydrogen bonds between water molecules are broken, they can be replaced by equivalent bonds. Web in the ammonia molecule group, the.
Properties of Water Presentation Biology
1 ammonia (nh 3) forms a weakly hydrogen. Web in the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair. Hydrogen bonding is the intermolecular forces acting between ammonia molecules. Web in ammonia (nh 3 ), the number of hydrogen bonds formed is limited since in each nitrogen.
Novel Technique Seamlessly Converts Ammonia to Green HydrogenUNIST News
The lone pair of electrons present in nitrogen is equal to one. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. However, we also know that. Of course it can interact with positively polarized hydrogens in an electrostatic way. 1 ammonia (nh 3) forms a weakly hydrogen.
9 Hydrogen Bond Examples in Real Life StudiousGuy
Theoretical studies of the reaction. Web and it has total three hydrogen bonding by the acceptance and donation. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. Web an ammonia molecule can donate and accept up to three hydrogen bonds. In the gaseous state at high temperature, the ammonia molecules have sufficient kinetic energy to overcome the attractions.
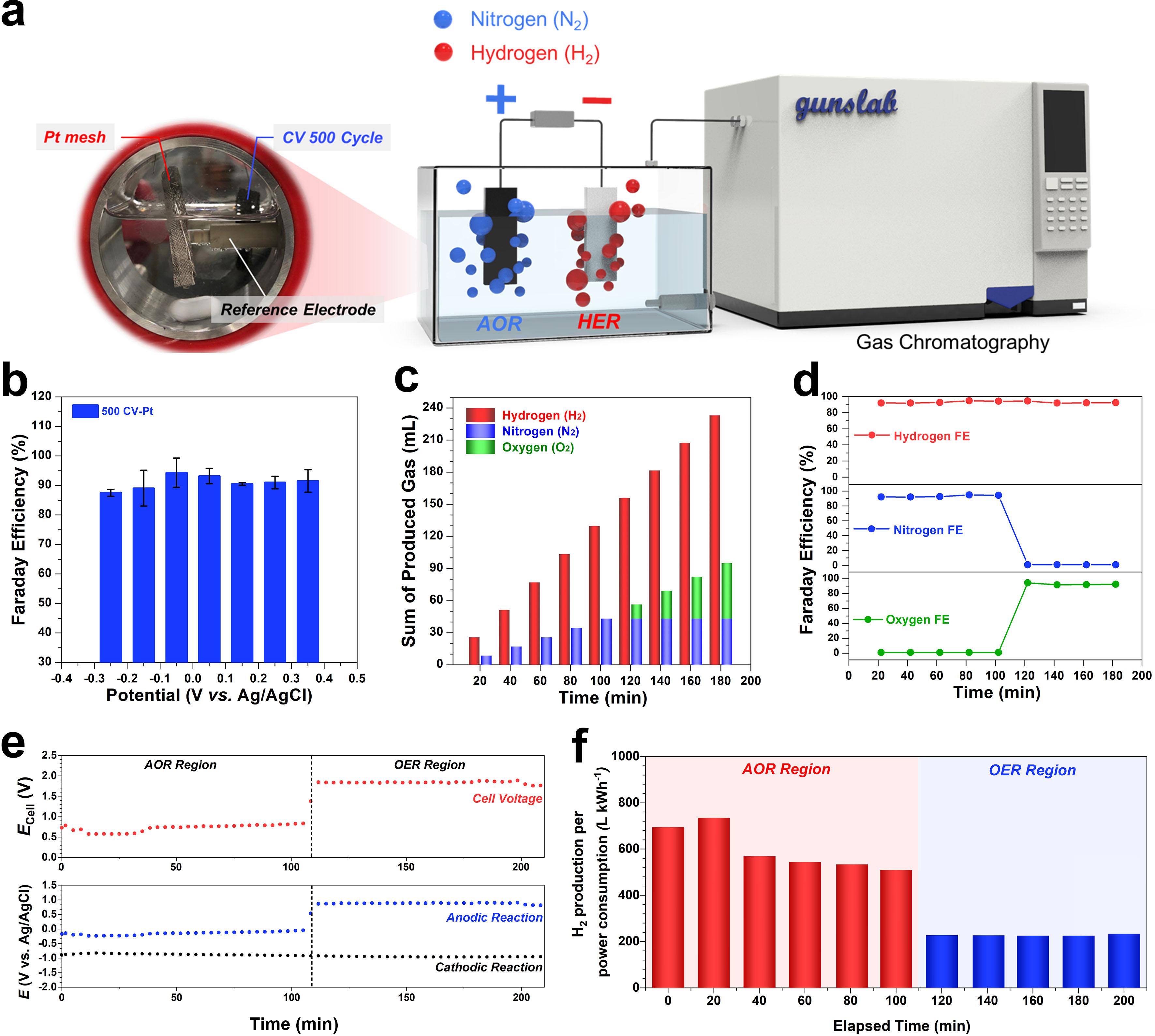
Tokyo Tech Breaking Ammonia A New Catalyst to Generate Hydrogen from
Theoretical studies of the reaction. When the hydrogen bonds between water molecules are broken, they can be replaced by equivalent bonds. Web actually, an ammonia molecule can form two hydrogen bonds, not one. Web and it has total three hydrogen bonding by the acceptance and donation. However, we also know that.
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG
However, we also know that. Web and it has total three hydrogen bonding by the acceptance and donation. Web ammonia can form four hydrogen bonds per molecule. Web in ammonia (nh 3 ), the number of hydrogen bonds formed is limited since in each nitrogen there is only one lone pair of electrons which is shared with one hydrogen. 1.
Ammonia Has Electronegative Atom Nitrogen Connected To Hydrogen.
Web ammonia is an important molecule due to its wide use in the fertiliser industry. Web in ammonia (nh 3 ), the number of hydrogen bonds formed is limited since in each nitrogen there is only one lone pair of electrons which is shared with one hydrogen. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. In some ways it makes.
In The Gaseous State At High Temperature, The Ammonia Molecules Have Sufficient Kinetic Energy To Overcome The Attractions To Other Ammonia Molecules.
1 ammonia (nh 3) forms a weakly hydrogen. In a group of ammonia molecules, there aren't enough. The lone pair on nitrogen can accept one hydrogen to form a hydrogen bond, and the three hydrogen. When the hydrogen bonds between water molecules are broken, they can be replaced by equivalent bonds.
The Lone Pair Of Electrons Present In Nitrogen Is Equal To One.
8.9k views 1 year ago. Web answer (1 of 3): The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more. Of course it can interact with positively polarized hydrogens in an electrostatic way.
To Understand Hydrogen Bonding In Ammonia (Nh3) We Need To Know That Ammonia Is A Polar Molecule.
Web in the ammonia molecule group, the lone pair of electrons is not enough for forming the hydrogen bond. Web actually, an ammonia molecule can form two hydrogen bonds, not one. Web in the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair. Theoretical studies of the reaction.



.PNG)