Chair Form Of Cyclohexane
Chair Form Of Cyclohexane - On careful examination of a chair conformation of cyclohexane, we find that the twelve hydrogens are not structurally equivalent. The chair structure of cyclohexane is considered to be the “perfect” conformation. Web two of the most common cyclohexane conformations are the chair and boat forms. The point group is d 3 d. Web the figure shows the symmetry elements in the chair form of cyclohexane. Web the chair conformation of cyclohexane. Table of contents conformations of cyclohexane boat and chair forms of cyclohexane Energies are 43 kj/mol (10 kcal/mol), 25 kj/mol (6 kcal/mol) and 21 kj/mol (5 kcal/mol). Web chair conformations of methyl cyclo hexane. Conformations of cycloalkanes drawing chair conformations monosubstituted cyclohexane disubstituted cyclohexane polysubstituted cyclohexane science > organic chemistry > alkanes, cycloalkanes, and functional.
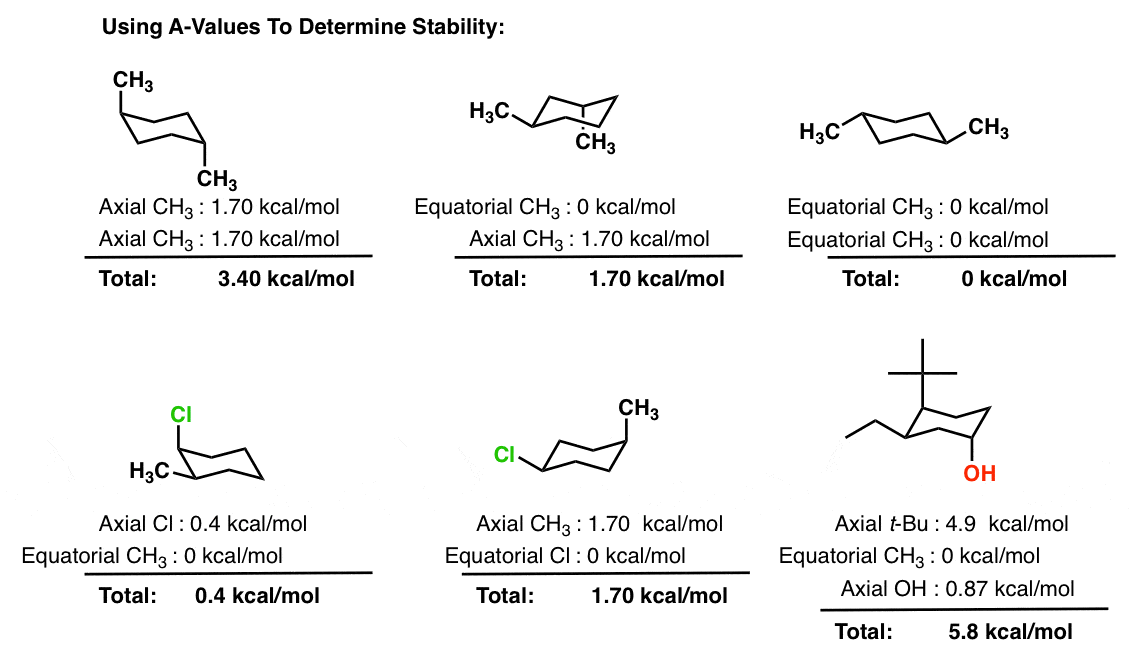
Web cyclohexane is formed by carbon and hydrogen atoms, structured in a hexagonal ring, with a chair conformation. Web chair conformations of methyl cyclo hexane. The axial positions point perpendicular to the plane of the ring, whereas the equatorial positions are around the. The mutual crowding of hydrogen atoms at carbons one and four further destabilise the boat conformation. Web the chair is the most stable of the three, owing to the staggered arrangement of all its links. Organic chemistry > unit 3 lesson 4: Web in an example of drawing chair conformation with constituents besides all hydrogens, like say an ethyl group and an isopropyl group, how do you determine the most stable confirmation? On careful examination of a chair conformation of cyclohexane, we find that the twelve hydrogens are not structurally equivalent. Web the chair conformation of cyclohexane. When it is observed at a 77°f, then around 99.99% of the cyclohexane molecules will take the form of this chair conformation.
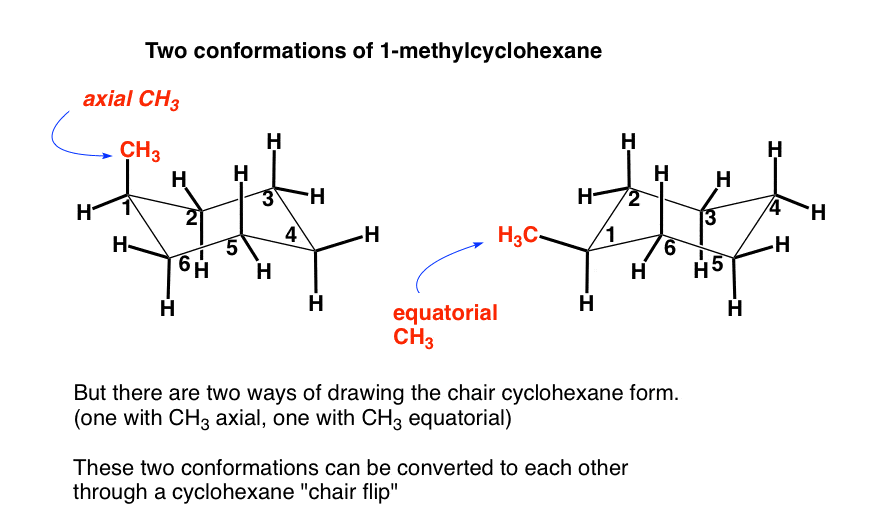
Web chair conformation of cyclohexane the flexibility of cyclohexane allows for a conformation which is almost free of ring strain. Web the chair conformation of cyclohexane is found to be the most stable conformer. The flexibility of cyclohexane allows for a conformation which is almost free of ring strain. Web in an example of drawing chair conformation with constituents besides all hydrogens, like say an ethyl group and an isopropyl group, how do you determine the most stable confirmation? Note that when the chair flips, the methyl group (shown in red) shifts between an equatorial position (on the left side of the equilibrium) and an axial position (on the right side of the equilibrium). On careful examination of a chair conformation of cyclohexane, we find that the twelve hydrogens are not structurally equivalent. In the structure below, the six red coloured bonds are axial and the six blue coloured bonds are equatorial. Web the chair conformation is the most stable conformation of cyclohexane. Web the figure shows the symmetry elements in the chair form of cyclohexane. • ( 4 votes) upvote downvote flag greekbutterfly 5 years ago
Phenol Structures Built with Orbit Model Set 68845NV
When it is observed at a 77°f, then around 99.99% of the cyclohexane molecules will take the form of this chair conformation. Web chair conformation of cyclohexane. Web cyclohexane is formed by carbon and hydrogen atoms, structured in a hexagonal ring, with a chair conformation. Web chair conformation of cyclohexane the flexibility of cyclohexane allows for a conformation which is.
Computer graphic of the chair form of cyclohexane Stock Image A705
Several million kilograms of cyclohexanone and cyclohexanol are produced annually. The mutual crowding of hydrogen atoms at carbons one and four further destabilise the boat conformation. Web the figure shows the symmetry elements in the chair form of cyclohexane. On careful examination of a chair conformation of cyclohexane, we find that the twelve hydrogens are not structurally equivalent. I n.
Cómo identificar formas cis y trans de ciclohexano [cerrado] Tiantan
Organic chemistry > unit 3 lesson 4: Web chair and boat shapes for cyclohexane double newman diagram for methylcyclohexane stability of cycloalkanes conformations of cyclohexane drawing chair conformations monosubstituted cyclohexane disubstituted cyclohexane polysubstituted cyclohexane science > organic chemistry > alkanes, cycloalkanes, and functional groups > I n chair cyclohexane there are two types of positions, axial and equatorial. The axial.
Chair Conformation of CyclohexaneV1 YouTube
Web the chair is the most stable of the three, owing to the staggered arrangement of all its links. Web the figure shows the symmetry elements in the chair form of cyclohexane. Web chair conformation of cyclohexane. The flexibility of cyclohexane allows for a conformation which is almost free of ring strain. Energies are 43 kj/mol (10 kcal/mol), 25 kj/mol.
Cyclohexane Chair Conformation Stability Which One Is Lower Energy?
The axial positions point perpendicular to the plane of the ring, whereas the equatorial positions are around the. Web cyclohexane is formed by carbon and hydrogen atoms, structured in a hexagonal ring, with a chair conformation. Table of contents conformations of cyclohexane boat and chair forms of cyclohexane Conformations of cycloalkanes drawing chair conformations monosubstituted cyclohexane disubstituted cyclohexane polysubstituted cyclohexane.
Chair Conformations of Cyclohexane equivalent structures? Physics
In this structure, all the carbon atoms are equivalent. On careful examination of a chair conformation of cyclohexane, we find that the twelve hydrogens are not structurally equivalent. Web cyclohexane is formed by carbon and hydrogen atoms, structured in a hexagonal ring, with a chair conformation. Web the chair is the most stable of the three, owing to the staggered.
MoleculAR an augmented reality app for organic chemistry Organic
The mutual crowding of hydrogen atoms at carbons one and four further destabilise the boat conformation. Web the chair conformation of cyclohexane. The flexibility of cyclohexane allows for a conformation which is almost free of ring strain. Of these three, the chair conformation is the most stable, while the boat conformation. Web cyclohexane and the chair structure:
Cyclohexane Chair Conformations Axial and Equatorial Tutorial Video
Web the chair is the most stable of the three, owing to the staggered arrangement of all its links. The axial positions point perpendicular to the plane of the ring, whereas the equatorial positions are around the. I n chair cyclohexane there are two types of positions, axial and equatorial. Web the chair conformation is the most stable conformation of.
The Cyclohexane Chair Flip Master Organic Chemistry
Web chair conformation of cyclohexane the flexibility of cyclohexane allows for a conformation which is almost free of ring strain. In this structure, all the carbon atoms are equivalent. The flexibility of cyclohexane allows for a conformation which is almost free of ring strain. The mutual crowding of hydrogen atoms at carbons one and four further destabilise the boat conformation..
How to Draw Cyclohexane Chair Conformations and Ring Flips YouTube
Web chair conformation of cyclohexane the flexibility of cyclohexane allows for a conformation which is almost free of ring strain. The point group is d 3 d. The flexibility of cyclohexane allows for a conformation which is almost free of ring strain. Web cyclohexane and the chair structure: Web the chair is the most stable of the three, owing to.
Web Chair Conformations Of Methyl Cyclo Hexane.
The point group is d 3 d. The chair structure of cyclohexane is considered to be the “perfect” conformation. Web two of the most common cyclohexane conformations are the chair and boat forms. Torsional strain destabilised the boat and skews conformations because the bonds are not perfectly staggered.
The Flexibility Of Cyclohexane Allows For A Conformation Which Is Almost Free Of Ring Strain.
I n chair cyclohexane there are two types of positions, axial and equatorial. Web chair conformation of cyclohexane the flexibility of cyclohexane allows for a conformation which is almost free of ring strain. Web cyclohexane and the chair structure: In this structure, all the carbon atoms are equivalent.
Web The Chair Is The Most Stable Of The Three, Owing To The Staggered Arrangement Of All Its Links.
In the structure below, the six red coloured bonds are axial and the six blue coloured bonds are equatorial. [9] it is used as a. Of these three, the chair conformation is the most stable, while the boat conformation. Web the figure shows the symmetry elements in the chair form of cyclohexane.
Web In An Example Of Drawing Chair Conformation With Constituents Besides All Hydrogens, Like Say An Ethyl Group And An Isopropyl Group, How Do You Determine The Most Stable Confirmation?
Web cyclohexane is formed by carbon and hydrogen atoms, structured in a hexagonal ring, with a chair conformation. Web chair and boat shapes for cyclohexane (video) | khan academy organic chemistry course: • ( 4 votes) upvote downvote flag greekbutterfly 5 years ago Web the chair conformation of cyclohexane.


![Cómo identificar formas cis y trans de ciclohexano [cerrado] Tiantan](https://i.stack.imgur.com/v3yX7.jpg)