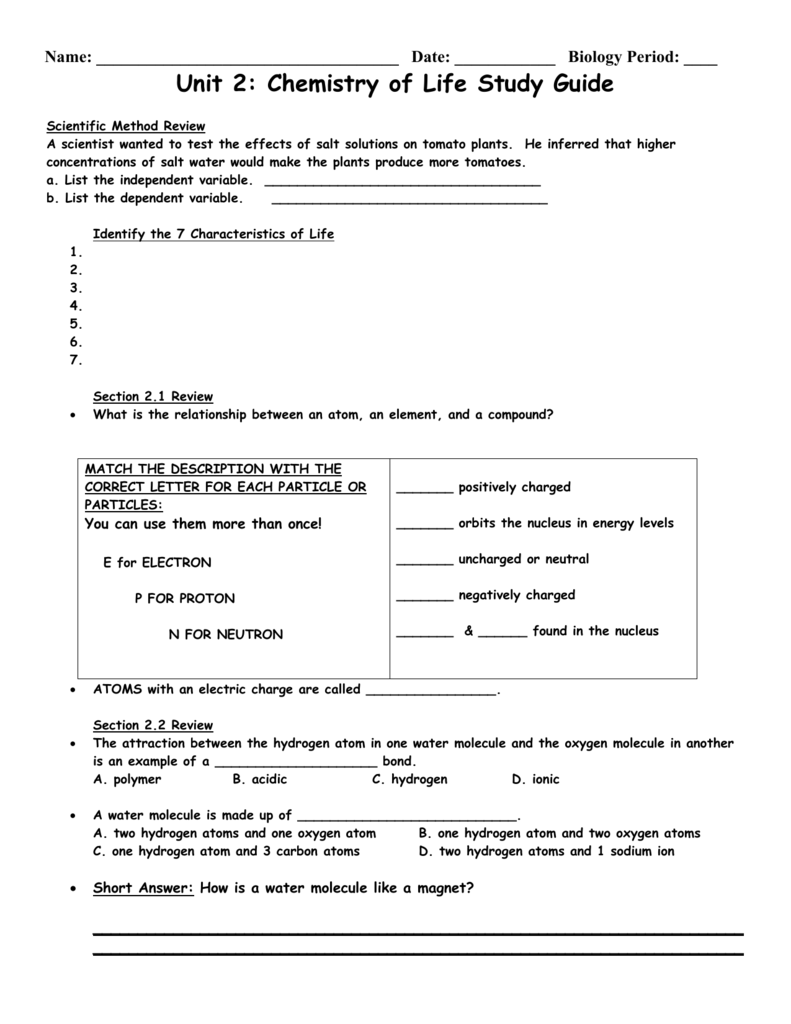
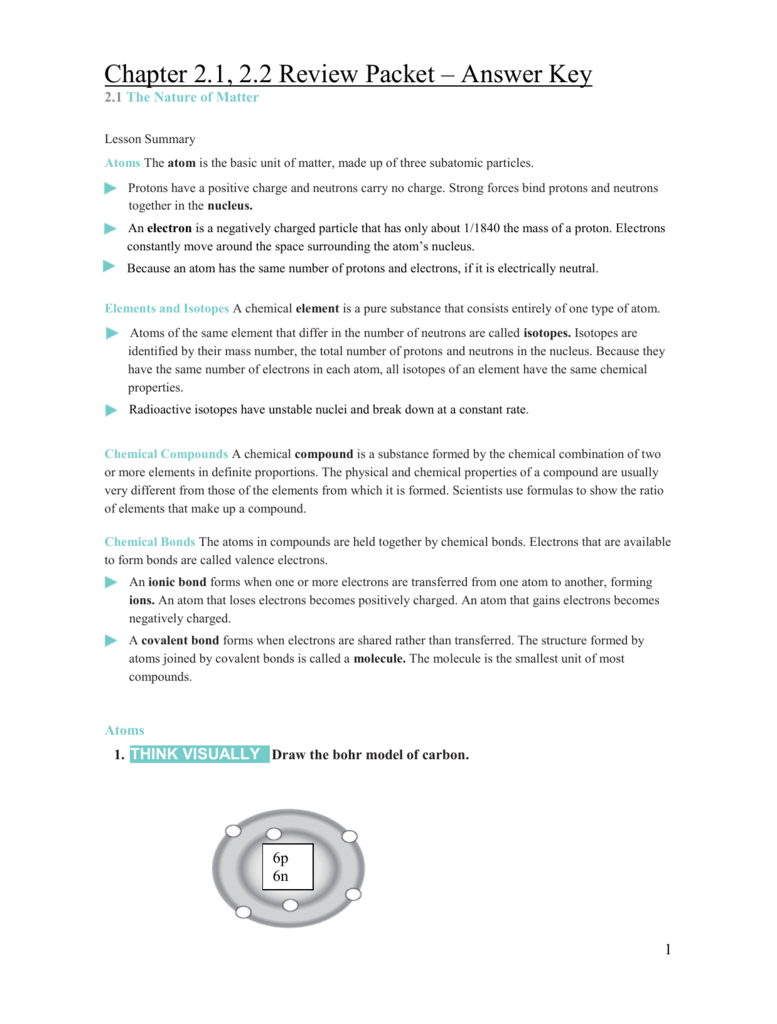
Chapter 2 The Chemistry Of Life Answer Key
Chapter 2 The Chemistry Of Life Answer Key - Electron orbitals and valence electrons 2.3. Web 2.1 the nature of matter what 3 subatomic particles make up atoms? Figure 2.1 foods such as bread, fruit, and cheese are rich sources of biological macromolecules. Web chapter 2 chemistry of life test answer key. The structure of the atom and isotopes 2.2. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus are the key. Chapter 2, the chemistry of life. An attraction between two oppositely charged ions, which form when one atom strips one or more electrons from another atom. This chapter provides the chemistry background needed to understand the human body, its functions, and its processes. The answer key will provide information about the structure of atoms, the different elements, and their.
Web here are some key concepts and answers that you may find in the answer key: Unit 1 introducing biology chapter 2 chemistry of life. By searching the title, publisher, or authors of guide you truly want, you can discover them rapidly. Web 1 / 83 flashcards learn test match created by abtastic2020 terms in this set (83) the three particles that make up an atom are a) protons, neutrons, and isotopes b) neutrons, isotopes and electrons c) positive, negatives, and electrons d) protons, neutrons, and electrons d (protons, neutrons, and electrons). It covers topics such as atoms, elements, and compounds. Web chapter 2 chemistry of life matter anything that takes up space an element is one of the basic building blocks of matter and cannot be broken down by chemical means. The basic unit of matter is called an. This is just one of the solutions for you to be successful. The chemistry of life review questions. Despite how small atoms are, they.
Because they have the same # of electrons what are the main types of chemical. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus are the key building blocks of the chemicals found in a sodium atom is a. This is just one of the solutions for you to be successful. Chapter 2 the chemistry of life reviewing key concepts answers. Unit 1 introducing biology chapter 2 chemistry of life. Protons neutrons electrons why do isotopes of the same element have the same chemical properties? Introduction to the chemistry of life. A 6 chapter 2 chemistry of life test answer key. Web chapter 2 chemistry of life test answer key. Electron orbitals and valence electrons 2.3.
Chapter 2 Chemical Basis Of Life Study Guide Answers Study Poster
Web chapter 2 introduces the molecular basis of life, and thereby advances students’ comprehension of the enduring understanding: This chapter provides the chemistry background needed to understand the human body, its functions, and its processes. The structure of the atom and isotopes 2.2. The chemistry of life review questions. Protons neutrons electrons why do isotopes of the same element have.
Chapter 2 The Chemistry Of Life Worksheet Answers worksheet
Web 2.1 the nature of matter what 3 subatomic particles make up atoms? Polarity in a water molecule is caused by. Electron orbitals and valence electrons 2.3. Answer key 1 chapter 2 chemistry of life test answer key. The chapter describes biochemical compounds and reactions as well as the significance of water to life.
Biology Study Guide Unit 2 Chemistry Of Life Study Poster
It covers topics such as atoms, elements, and compounds. Web chapter 2 chemistry of life matter anything that takes up space an element is one of the basic building blocks of matter and cannot be broken down by chemical means. Unit 1 introducing biology chapter 2 chemistry of life. By searching the title, publisher, or authors of guide you truly.
chapter 2 The chemistry of life Crossword WordMint
This is just one of the solutions for you to be successful. Chapter 1, the science of biology. An attraction between two oppositely charged ions, which form when one atom strips one or more electrons from another atom. Web chapter 2 introduces the molecular basis of life, and thereby advances students’ comprehension of the enduring understanding: Click the card to.
Biology Chapter 2 The Chemistry Of Life Worksheet Answers —
The building blocks of life. Web here are some key concepts and answers that you may find in the answer key: Unit 1 introducing biology chapter 2 chemistry of life. Web 2.1 the nature of matter what 3 subatomic particles make up atoms? The first section of the chapter 2 answer key focuses on the building blocks of life.
chapter 2 The chemistry of life Crossword WordMint
Polarity in a water molecule is caused by. An attraction between two oppositely charged ions, which form when one atom strips one or more electrons from another atom. Introduction to the chemistry of life. The structure of the atom and isotopes 2.2. Web chapter 2 chemistry of life test answer key.
Units/Chapters
Answer key 1 chapter 2 chemistry of life test answer key. Web chapter 2 the chemistry of life study guide terms in this set (85) the main source of energy for living things carbohydrates help carry out chemical reactions proteins contain hydrogen, oxygen, nitrogen,. An attraction between two oppositely charged ions, which form when one atom strips one or more.
PPT Biology Biochemistry Unit Chapter 2 The Chemistry of Life
It covers topics such as atoms, elements, and compounds. Web 2.1 the nature of matter what 3 subatomic particles make up atoms? Polarity in a water molecule is caused by. By searching the title, publisher, or authors of guide you truly want, you can discover them rapidly. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus are the key.
Chapter 2 The Chemistry Of Life Section 2.1 The Nature Of Matter Answer
Protons neutrons electrons why do isotopes of the same element have the same chemical properties? Introduction to the chemistry of life. Solutes dissolve in a solvent 16 2. Polarity in a water molecule is caused by. The building blocks of life.
Biology Chapter 2 The Chemistry Of Life Study Guide Study Poster
The structure of the atom and isotopes 2.2. By searching the title, publisher, or authors of guide you truly want, you can discover them rapidly. It is even more surprising that that over 90% of the. Click the card to flip 👆. This chapter provides the chemistry background needed to understand the human body, its functions, and its processes.
An Attraction Between Two Oppositely Charged Ions, Which Form When One Atom Strips One Or More Electrons From Another Atom.
The chemistry of life review questions. The covalent bond introduction matter is defined as anything that takes up space and has mass and so. Protons neutrons electrons why do isotopes of the same element have the same chemical properties? Chapter 2, the chemistry of life.
A 6 Chapter 2 Chemistry Of Life Test Answer Key.
It covers topics such as atoms, elements, and compounds. The basic unit of matter is called an. The first section of the chapter 2 answer key focuses on the building blocks of life. Web 1 / 83 flashcards learn test match created by abtastic2020 terms in this set (83) the three particles that make up an atom are a) protons, neutrons, and isotopes b) neutrons, isotopes and electrons c) positive, negatives, and electrons d) protons, neutrons, and electrons d (protons, neutrons, and electrons).
Polarity In A Water Molecule Is Caused By.
The structure of the atom and isotopes 2.2. Electron orbitals and valence electrons 2.3. Figure 2.1 foods such as bread, fruit, and cheese are rich sources of biological macromolecules. Chapter 2 the chemistry of life reviewing key concepts answers.
The Building Blocks Of Life.
Web chapter 2 introduces the molecular basis of life, and thereby advances students’ comprehension of the enduring understanding: The chapter describes biochemical compounds and reactions as well as the significance of water to life. Unit 1 introducing biology chapter 2 chemistry of life. It is even more surprising that that over 90% of the.