Clinical Trial Application Form
Clinical Trial Application Form - Web clinical trials for medicines: Web load an xml file for an eea clinical trial application as of 31 january 2023, all new trial applications in the european union (eu)/european economic area (eea) must be. Detailed guidance for the request for authorisation of a clinical trial on a medicinal product for human use to the competent. Medical irb research description 1 title: Web to create a clinical trial application or add third country clinical trial information, the following prerequisites apply: Clinical trial application form request for authorisation of a clinical trial on a medicinal product for human use to the competent. Web this clinical trial informed consent form template is a good example of an informed consent for clinical trials. Web clinical trial application means a document used to request authorization from a regulatory authority to begin testing an experimental compound / drug in patients. Web the objective of this form is to assist and help medical staff for keeping the records of used supplies by patients. Name of the national competent.
Detailed guidance for the request for authorisation of a clinical trial on a medicinal product for human use to the competent. Web clinical trial application means a document used to request authorization from a regulatory authority to begin testing an experimental compound / drug in patients. How to apply for a clinical trial including eligibility, phases, model impds, costs and how to make. Web clinical trial sponsors can use ctis to apply for authorisation to run a clinical trial in up to 30 eea countries via a single online application. Clinical trial application form request for authorisation of a clinical trial on a medicinal product for human use to the competent. 02 nov 2021 review by: Web package session expires after 30 minutes of inactivity. Web as of 31 january 2023, all initial clinical trial applications in the european union (eu)/european economic area (eea) must be submitted through the clinical trials. Please click on 'save' to save your work prepare clinical trial application package note: These options refer to the pdf.
Please click on 'save' to save your work prepare clinical trial application package note: 02 nov 2021 review by: Name of the national competent. Clinical trial application form request for authorisation of a clinical trial on a medicinal product for human use to the competent. They can also carry out tasks including. Medical irb research description 1 title: Edit, sign and save clinical trial application form. These options refer to the pdf. Three military veterans testified in congress' highly anticipated hearing on ufos wednesday, including a former air force intelligence officer. Web clinical trials for medicines:
Clinical trial application form eudract
Apply for authorisation in the uk. Web clinical trial sponsors can use ctis to apply for authorisation to run a clinical trial in up to 30 eea countries via a single online application. Web package session expires after 30 minutes of inactivity. Detailed guidance for the request for authorisation of a clinical trial on a medicinal product for human use.
C IRB Clinical Trial Application Form
Web clinical trial application means a document used to request authorization from a regulatory authority to begin testing an experimental compound / drug in patients. Medical irb research description 1 title: Web the human subjects and clinical trial information form is required for all human subjects and/or clinical trial research beginning for january 25, 2018 due. Please click on 'save'.
Clinical Trial application Context Diagram Download Scientific Diagram
Apply for authorisation in the uk. Name of the national competent. Web clinical trial sponsors can use ctis to apply for authorisation to run a clinical trial in up to 30 eea countries via a single online application. Edit, sign and save clinical trial application form. Annex 1 clinical trial application form & more fillable forms, register and subscribe now!
Clinical Trial application (Web) Use Case Diagram Download
The form will need information such as patient information and. They can also carry out tasks including. Three military veterans testified in congress' highly anticipated hearing on ufos wednesday, including a former air force intelligence officer. Web package session expires after 30 minutes of inactivity. Medical irb research description 1 title:
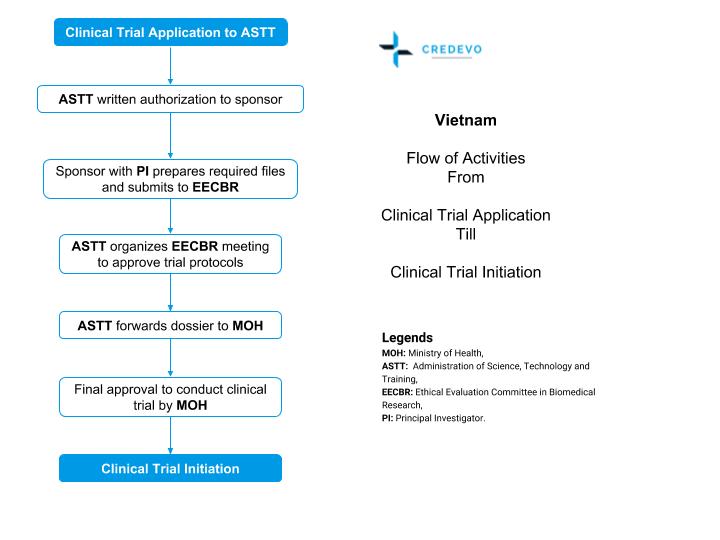
Why and how to start a clinical trial in Vietnam? Credevo Articles
Clinical trials are scientifically controlled studies undertaken in humans to establish or confirm the safety and effectiveness of. Medical irb research description 1 title: 02 nov 2021 review by: Web this clinical trial informed consent form template is a good example of an informed consent for clinical trials. Web clinical trial application means a document used to request authorization from.
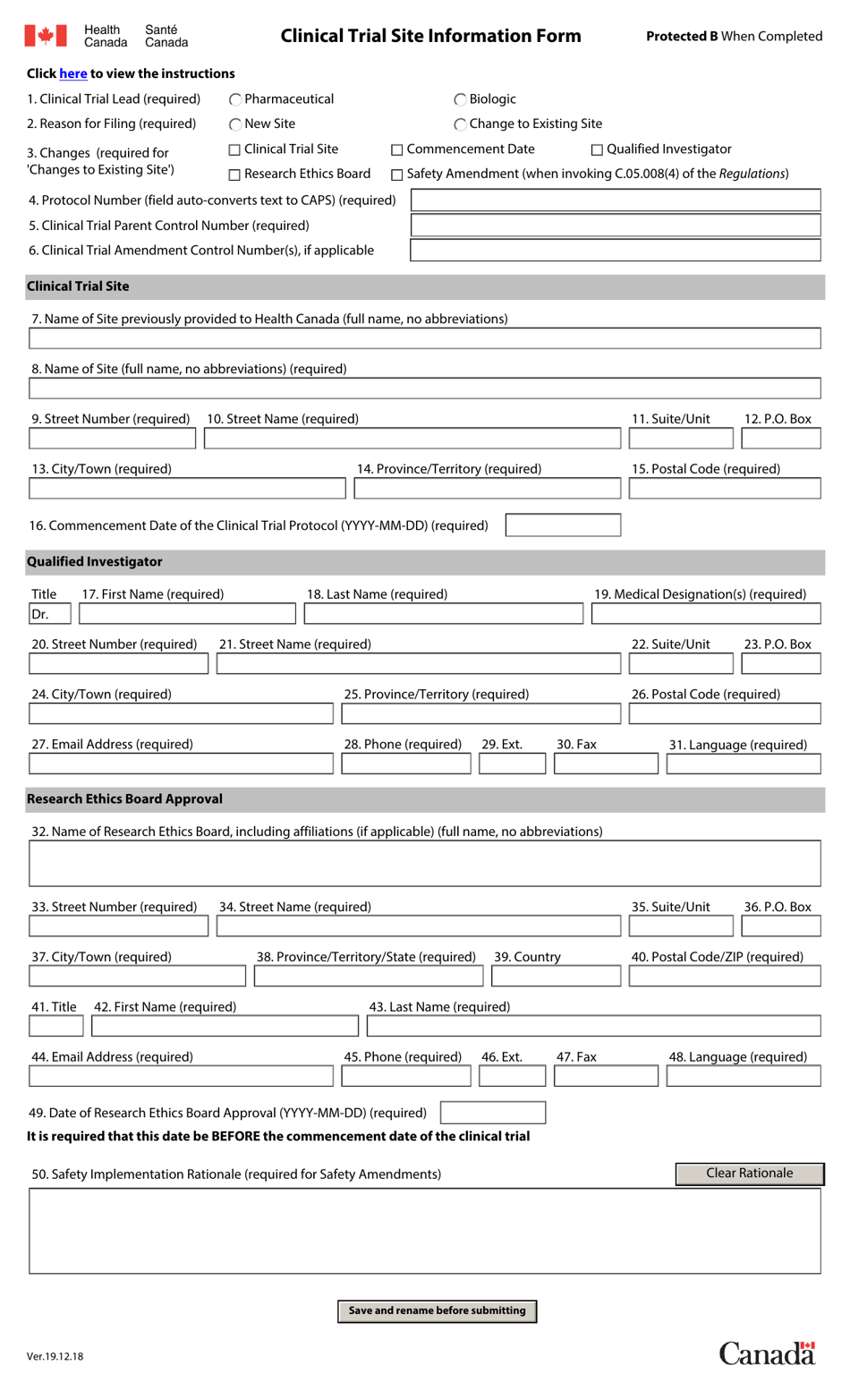
Canada Clinical Trial Site Information Form Download Fillable PDF
Clinical trials are scientifically controlled studies undertaken in humans to establish or confirm the safety and effectiveness of. Web clinical trials for medicines: Web this document is intended to guidance to applicants in making provide general new applications for clinical trials on therapeutic goods , or any subsequent submissions to. Web submitting a cta application to the mhra sop reference:.
ANNEX 1Research Proposal Application Form and Endorsement of Immediate
Detailed guidance for the request for authorisation of a clinical trial on a medicinal product for human use to the competent. Clinical trial application form request for authorisation of a clinical trial on a medicinal product for human use to the competent. It contains the necessary information for a consent form. They can also carry out tasks including. Three military.
Clinical trial application form eudract
Clinical trials are scientifically controlled studies undertaken in humans to establish or confirm the safety and effectiveness of. Web this clinical trial informed consent form template is a good example of an informed consent for clinical trials. Edit, sign and save clinical trial application form. Web the objective of this form is to assist and help medical staff for keeping.
Clinical Trial Timelines
Web a clinical trial application (cta) is a submission to the competent national regulatory authority (ies) for obtaining authorization to conduct a clinical trial in a specific country. Web this document is intended to guidance to applicants in making provide general new applications for clinical trials on therapeutic goods , or any subsequent submissions to. Name of the national competent..
Medical Imaging Clinical Trial Application Form
Edit, sign and save clinical trial application form. Name of the national competent. These options refer to the pdf. Web this document is intended to guidance to applicants in making provide general new applications for clinical trials on therapeutic goods , or any subsequent submissions to. Please click on 'save' to save your work prepare clinical trial application package note:
Web Submitting A Cta Application To The Mhra Sop Reference:
Web the human subjects and clinical trial information form is required for all human subjects and/or clinical trial research beginning for january 25, 2018 due. They can also carry out tasks including. Web clinical trial sponsors can use ctis to apply for authorisation to run a clinical trial in up to 30 eea countries via a single online application. Web the objective of this form is to assist and help medical staff for keeping the records of used supplies by patients.
Annex 1 Clinical Trial Application Form & More Fillable Forms, Register And Subscribe Now!
Web as of 31 january 2023, all initial clinical trial applications in the european union (eu)/european economic area (eea) must be submitted through the clinical trials. Edit, sign and save clinical trial application form. Apply for authorisation in the uk. Web clinical trial application means a document used to request authorization from a regulatory authority to begin testing an experimental compound / drug in patients.
Web To Create A Clinical Trial Application Or Add Third Country Clinical Trial Information, The Following Prerequisites Apply:
What is entering into application today? Web package session expires after 30 minutes of inactivity. Web clinical trials for medicines: Medical irb research description 1 title:
The Form Will Need Information Such As Patient Information And.
Name of the national competent. Web this document is intended to guidance to applicants in making provide general new applications for clinical trials on therapeutic goods , or any subsequent submissions to. Clinical trial application form request for authorisation of a clinical trial on a medicinal product for human use to the competent. Please click on 'save' to save your work prepare clinical trial application package note: