Does Oxygen And Bromine Form An Ionic Compound
Does Oxygen And Bromine Form An Ionic Compound - An oxygen atom gains two electrons to form an oxide ion. Both oxygen and bronie are nonmetals. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. 2 does barium and oxygen form an ionic compound? Web in covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals. To name an ionic compound, the. Web define ionic and molecular (covalent) compounds. Web there are two ways to recognize ionic compounds. However they from a covalent bond.
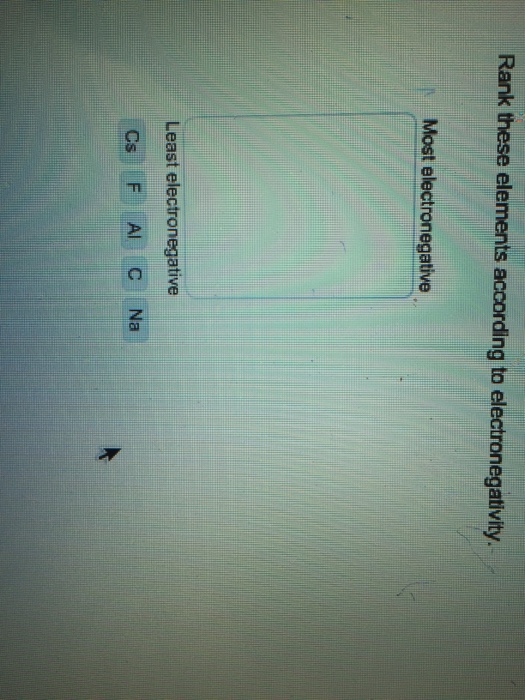
First, compounds between metal and nonmetal elements are usually ionic. Predict the type of compound formed from elements based on their location within the periodic table. Web up to $3 cash back 33. 3 can oxygen bond to bromine? Although both of these ions have. Web bromine dioxide (bro 2) bromine can form several different oxides : Write a formula for the ionic compound that forms between each pair of elements. Web article talk read edit view history bromine compounds are compounds containing the element bromine (br). Web there are two ways to recognize ionic compounds. Web in principle, iodine oxide (io) can also be measured by the chemical conversion resonance fluorescence method, but this has yet to be done in the atmosphere.
Both oxygen and bronie are nonmetals. An example of a covalent compound is ammonia. Web bromine dioxide (bro 2) bromine can form several different oxides : Web an ionic compound is made up of charged particles, called ions. As a general rule, nonmetals will form covalent bonds with. Dibromine monoxide (br 2 o) bromine dioxide (bro 2) dibromine trioxide (br 2 o 3) dibromine pentoxide (br. Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals. 3 can oxygen bond to bromine? Web there are two ways to recognize ionic compounds. Web by ibanth contents hide 1 is oxygen and bromine ionic or covalent?
Solved Add electron dots and charges as necessary to show
Predict the type of compound formed from elements based on their location within the periodic table. Web there are two ways to recognize ionic compounds. Web from numbers of equivalent portions of acid bromine formed from the previous reaction, the ratio between oxygen and bromine was calculated, with the exact value of o:br. However they from a covalent bond. Web.
science exothermic reaction sodium bromine Fundamental Photographs
Web up to $3 cash back 33. Ions are formed by the transfer of electrons. Write a formula for the ionic compound that forms between each pair of elements. Web an ionic compound is made up of charged particles, called ions. Web article talk read edit view history bromine compounds are compounds containing the element bromine (br).
Chemical Elements Bromine
Web in principle, iodine oxide (io) can also be measured by the chemical conversion resonance fluorescence method, but this has yet to be done in the atmosphere. Web an ionic compound is made up of charged particles, called ions. Dibromine monoxide (br 2 o) bromine dioxide (bro 2) dibromine trioxide (br 2 o 3) dibromine pentoxide (br. It was first.
6. The most common ion of bromine has a charge of A. +2 В. +1 C
To name an ionic compound, the. Although both of these ions have. An example of a covalent compound is ammonia. Web bromine dioxide (bro 2) bromine can form several different oxides : Web an ionic compound is made up of charged particles, called ions.
Chemical Elements Bromine
Web from numbers of equivalent portions of acid bromine formed from the previous reaction, the ratio between oxygen and bromine was calculated, with the exact value of o:br. For example, cabr 2 contains a. Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals..
Bromine Form Periodic Table Of Elements Stock Illustration Image 7137169
Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals. As a general rule, nonmetals will form covalent bonds with. Web bromine dioxide (bro 2) bromine can form several different oxides : Ions are formed by the transfer of electrons. First, compounds between metal.
Chemistry experiment 32 Reaction between bromine and aluminium YouTube
Web in covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Both oxygen and bronie are nonmetals. Ions are formed by the transfer of electrons. Web from numbers of equivalent portions of acid bromine formed from the previous reaction, the ratio between oxygen and bromine was calculated, with the exact value of.
Understanding Ionic Bond Formation Between Manganese And Chlorine
An example of a covalent compound is ammonia. Both oxygen and bronie are nonmetals. Web define ionic and molecular (covalent) compounds. Web bromine dioxide (bro 2) bromine can form several different oxides : However they from a covalent bond.
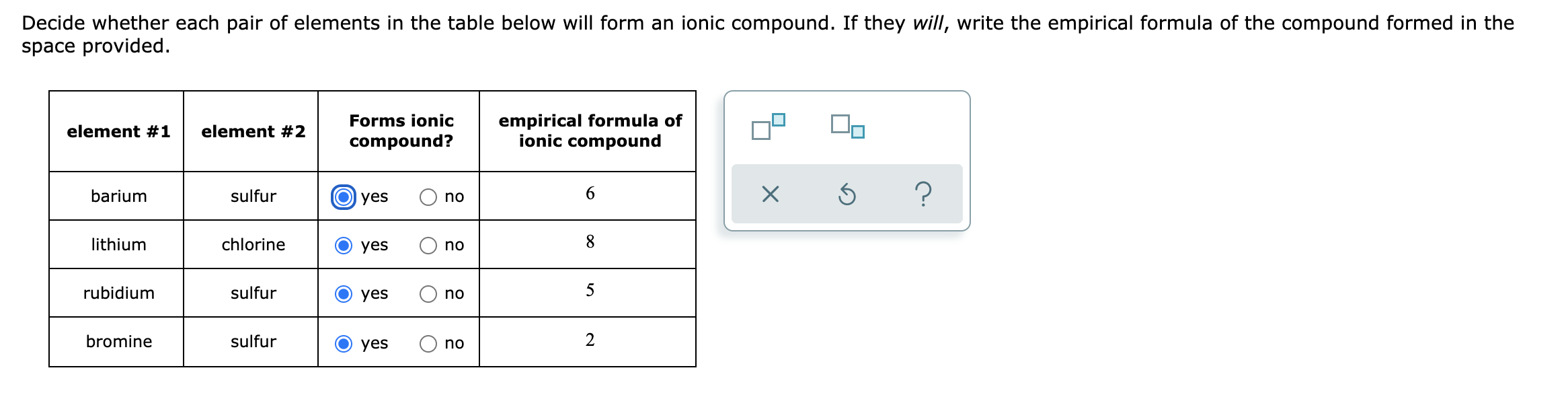
Answered Decide whether each pair of elements in… bartleby
Web article talk read edit view history bromine compounds are compounds containing the element bromine (br). Web from numbers of equivalent portions of acid bromine formed from the previous reaction, the ratio between oxygen and bromine was calculated, with the exact value of o:br. Web up to $3 cash back 33. Web in principle, iodine oxide (io) can also be.
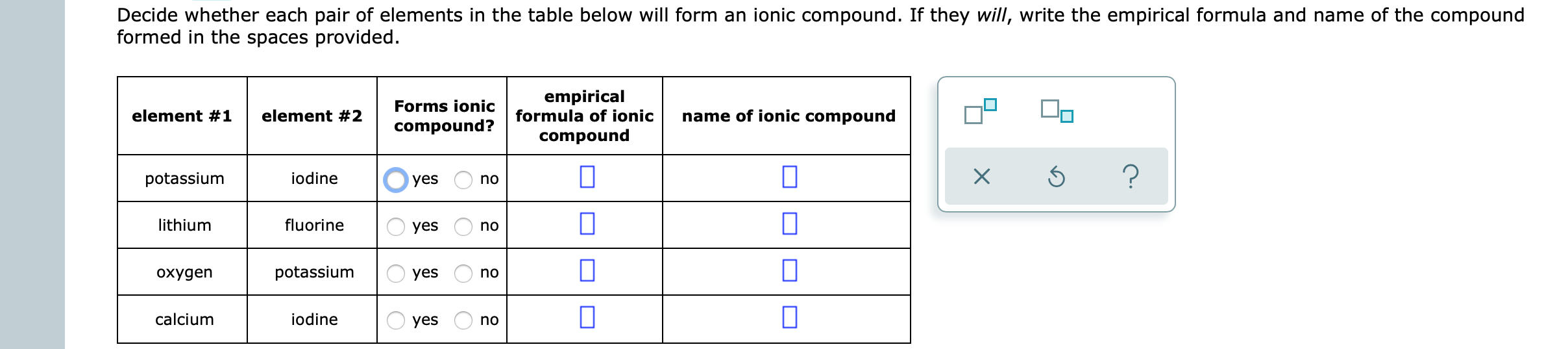
Answered Decide whether each pair of elements in… bartleby
Web up to $3 cash back 33. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals. Predict.
Web An Ionic Compound Is Made Up Of Charged Particles, Called Ions.
Web by ibanth contents hide 1 is oxygen and bromine ionic or covalent? For example, cabr 2 contains a. Web up to $3 cash back 33. Web article talk read edit view history bromine compounds are compounds containing the element bromine (br).
First, Compounds Between Metal And Nonmetal Elements Are Usually Ionic.
Predict the type of compound formed from elements based on their location within the periodic table. 2 does barium and oxygen form an ionic compound? Web bromine is a chemical element with the symbol br and atomic number 35. Web up to 6% cash back the compound contains a metal (potassium) and a nonmetal (oxygen), so it is an ionic compound, with no transition metals.
Web Bromine Dioxide Is The Chemical Compound Composed Of Bromine And Oxygen With The Formula Bro 2.
To name an ionic compound, the. 3 can oxygen bond to bromine? Web no it doesn't. As a general rule, nonmetals will form covalent bonds with.
Web In Covalent Compounds, Atoms Form Covalent Bonds That Consist Of Electron Pairs Shared Between Two Adjacent Atomic Nuclei.
Dibromine monoxide (br 2 o) bromine dioxide (bro 2) dibromine trioxide (br 2 o 3) dibromine pentoxide (br. Web there are two ways to recognize ionic compounds. Although both of these ions have. Ions are formed by the transfer of electrons.