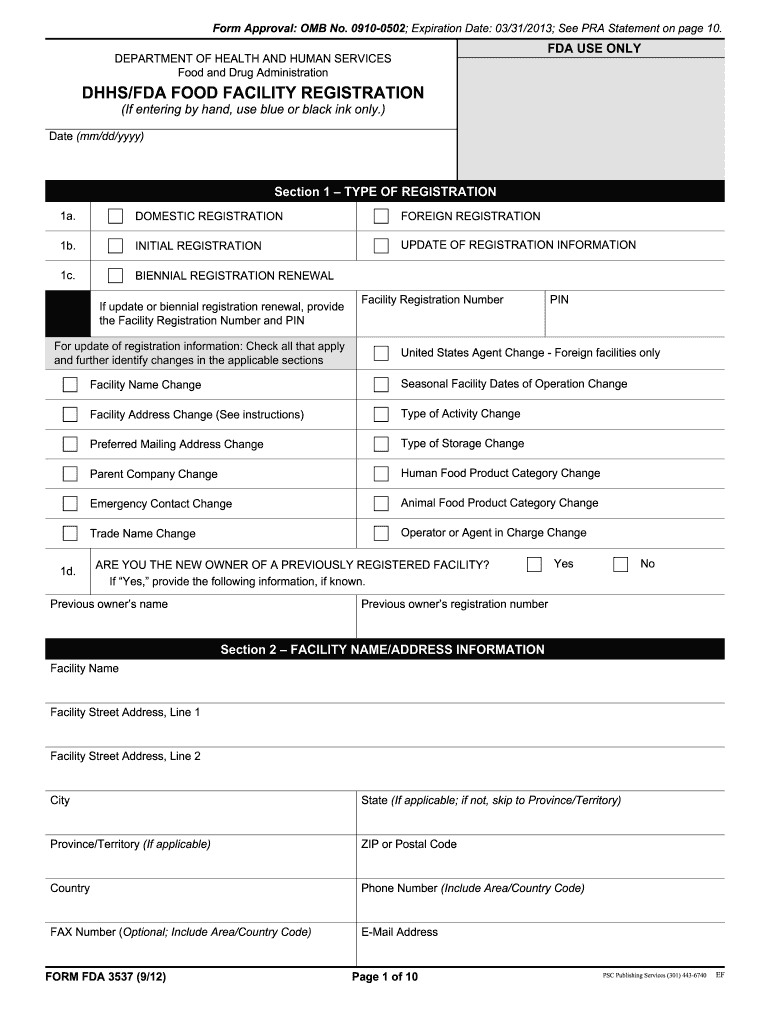
Fda Form 3537
Fda Form 3537 - Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Online registration of food facilities. Create a new form 3537 please read and understand the information below. While the fda expects all registrants to provide their duns number with their registration or. If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international). Web omb burden statement fda form 3537/3537a. You may obtain a copy of this form by writing to the u.s. Field appears upon user selecting ‘yes’ option. Instructions are available on the form. Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537.
Field appears upon user selecting ‘yes’ option. You may obtain a copy of this form by writing to the u.s. Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Agent identification number in section 7; Create a new form 3537 please read and understand the information below. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Web omb burden statement fda form 3537/3537a. Use the following instructions to download the form if you encounter an. Addition of unique facility identifier (ufi) field in section 2. If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international).
If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international). Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537. Online registration of food facilities. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Use the following instructions to download the form if you encounter an. Fda strongly encourages electronic registration via the internet, which. You may obtain a copy of this form by writing to the u.s. Create a new form 3537 please read and understand the information below. Agent identification number in section 7; Web omb burden statement fda form 3537/3537a.
Form FDA 3537 Fill Out and Sign Printable PDF Template signNow
Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Field appears upon user selecting ‘yes’ option. While the fda expects all registrants to provide their duns number with their registration or. You may obtain a copy of this form by writing to the u.s. Web omb burden statement fda form 3537/3537a.
36 Fda Forms And Templates free to download in PDF
Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Field appears upon user selecting ‘yes’ option. Online registration of food facilities. Use the following instructions to download.
Menlo Park Software 100 Perfect Compliance
Field appears upon user selecting ‘yes’ option. While the fda expects all registrants to provide their duns number with their registration or. Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Create a new form 3537 please read and understand the information below. Fda strongly encourages.
Form FDA 3356 Establishment Registration and Listing for HCT/Ps Free
While the fda expects all registrants to provide their duns number with their registration or. Instructions are available on the form. Create a new form 3537 please read and understand the information below. Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Online registration of food.
Form FDA 3632 Product Reports on Lasers and Products Containing
Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Web omb burden statement fda form 3537/3537a. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Use the following instructions to download the form if you encounter an. Agent identification number in.
Why is the 2253 FDA Form Comments Field Not Populated in Vault? Veeva
Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537. While the fda expects all registrants to provide their duns.
Form FDA 3537a DHHS/FDA Cancellation of Food Facility Registration
Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537. While the fda expects all registrants to provide their duns.
FDA FORM 3514 PDF
If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international). Addition of unique facility identifier (ufi) field in section 2. Use the following instructions to download the form if you encounter an. Food and drug administration, center for food safety and applied.
Fda 766 Fill Online, Printable, Fillable, Blank pdfFiller
Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Online registration of food facilities. Field appears upon user selecting ‘yes’ option. Instructions are available on the form. While the fda expects all registrants to provide their duns number with their registration or.
Instrucciones FORM 3537 Instructions for Filling Out DHHS/FDA Forms
Web omb burden statement fda form 3537/3537a. Instructions are available on the form. Use the following instructions to download the form if you encounter an. Online registration of food facilities. Agent identification number in section 7;
Create A New Form 3537 Please Read And Understand The Information Below.
Depending on the browser you are using, you may need to download the form to enable field fillable functionality. You may obtain a copy of this form by writing to the u.s. Fda strongly encourages electronic registration via the internet, which. Instructions are available on the form.
Web If You Submit A Registration Or Registration Renewal By Mail Or Fax, You Must Use The Paper Version Of Form Fda 3537.
Web omb burden statement fda form 3537/3537a. Online registration of food facilities. Agent identification number in section 7; Field appears upon user selecting ‘yes’ option.
While The Fda Expects All Registrants To Provide Their Duns Number With Their Registration Or.
Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Use the following instructions to download the form if you encounter an. Addition of unique facility identifier (ufi) field in section 2.