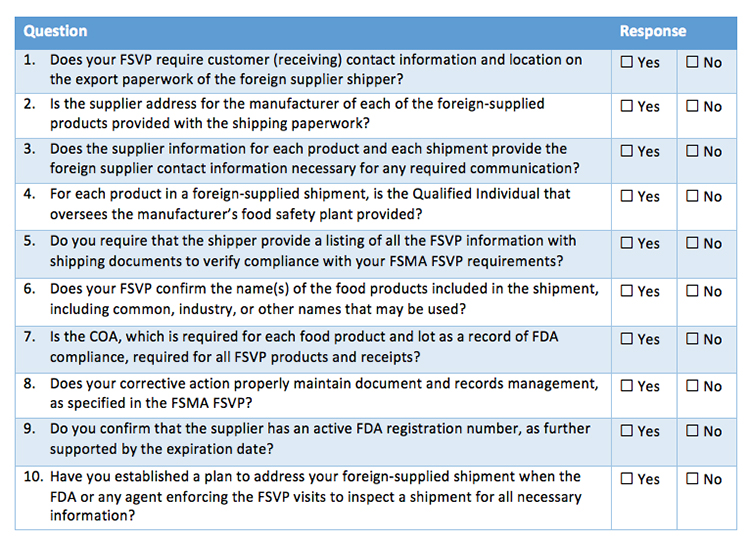
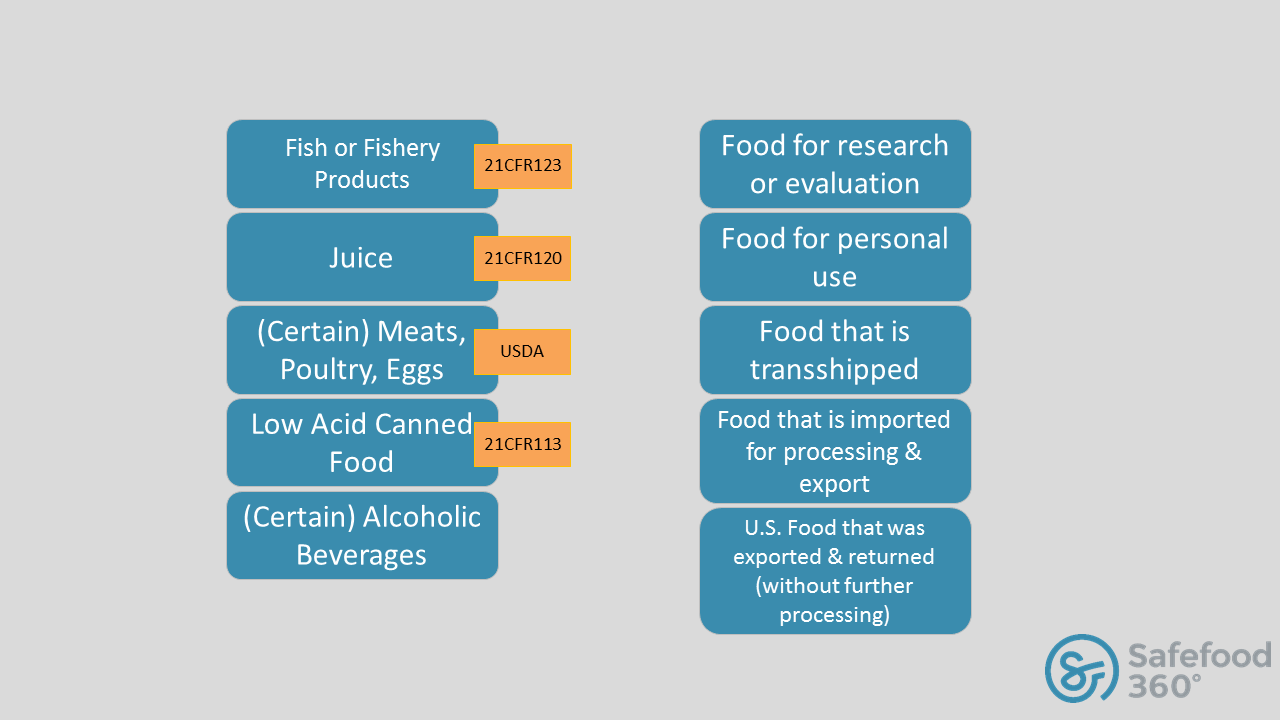
Fsvp Foreign Supplier Evaluation Form
Fsvp Foreign Supplier Evaluation Form - Customs and border protection see more on the. Web the foreign suppliers verification programs (fsvp) importer portal for fsvp records submission is a means for importers to upload fsvp records. Web the food and drug administration (fda) is adopting a regulation on foreign supplier verification programs (fsvps) for importers of food for humans and animals. Web approved foreign suppliers (with limited exceptions). Web evaluate risks posed by the food and the performance of the foreign supplier, considering: Web for each entry line of food subject to the foreign supplier verification program (fsvp), offered for importation into the united states, you must ensure that. Web if your facility is subject to fsvp, the food and drug administration (fda) has now released a document for foreign supplier verification programs for importers. O the hazard analysis for the food; Food and drug administration (fda). Determining the fsvp importer ;
Web fsvp foreign supplier evaluation form rating ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ 4.8 satisfied 20 votes how to fill out and sign fsvp program template online? Web fsvp is a program that importers covered by the rule must have in place to verify that their foreign suppliers are producing food in a manner that provides the same level of public. Web policy regarding certain entities subject to the current good manufacturing practice and preventive controls, produce safety, and/or foreign supplier verification. Determine and apply appropriate verification activities and assess results. Web supplier, the default verification procedure is the performance of a properly conducted onsite audit of the foreign supplier before initially importing the food and at least. Food and drug administration (fda). Web the food and drug administration (fda) is adopting a regulation on foreign supplier verification programs (fsvps) for importers of food for humans and animals. O the entity that will be applying hazard controls, such. Web in accordance with section 805 (g) of the federal food, drug, and cosmetic act (21 u.s.c. Web this is commonly achieved by reviewing the foreign supplier's food safety records, auditing reports, and performance past.
Customs and border protection see more on the. Web for each entry line of food subject to the foreign supplier verification program (fsvp), offered for importation into the united states, you must ensure that. Web importers covered by the fsvp regulation must put in place foreign supplier verification programs (fsvps) to verify that their foreign suppliers are producing food using. Web this is commonly achieved by reviewing the foreign supplier's food safety records, auditing reports, and performance past. Determine and apply appropriate verification activities and assess results. 384a (g)), we are publishing a list on our website of importers subject to. Web if your facility is subject to fsvp, the food and drug administration (fda) has now released a document for foreign supplier verification programs for importers. Web supplier, the default verification procedure is the performance of a properly conducted onsite audit of the foreign supplier before initially importing the food and at least. Food importers have required to verify. Web as an importer, once you have completed foreign supplier verification programs (fsvp) for each of your food items from your foreign suppliers, you can file.
FSMA Checklist Foreign Supplier Verification Program Requirements
O the entity that will be applying hazard controls, such. Implement corrective action(s), if needed. Web the foreign suppliers verification programs (fsvp) importer portal for fsvp records submission is a means for importers to upload fsvp records. Web supplier, the default verification procedure is the performance of a properly conducted onsite audit of the foreign supplier before initially importing the.
Get FSVP Compliant with Sirocco Consulting! Sirocco
Food importers have required to verify. Web in accordance with section 805 (g) of the federal food, drug, and cosmetic act (21 u.s.c. Consumers, the foreign supplier verification programs (fsvp) developed a rule titled importers of food for human and animal. 384a (g)), we are publishing a list on our website of importers subject to. Web fsvp is a program.
FSMA Final Rules Foreign Supplier Verification Program (FSVP) for
Determining the fsvp importer ; Web approved foreign suppliers (with limited exceptions). Web in accordance with section 805 (g) of the federal food, drug, and cosmetic act (21 u.s.c. Determine and apply appropriate verification activities and assess results. Web the food and drug administration (fda) is adopting a regulation on foreign supplier verification programs (fsvps) for importers of food for.
Foreign Supplier Verification Programs (FSVP) for Importers Global
Web the foreign suppliers verification programs (fsvp) importer portal for fsvp records submission is a means for importers to upload fsvp records. Web the food and drug administration (fda) is adopting a regulation on foreign supplier verification programs (fsvps) for importers of food for humans and animals. Web if your facility is subject to fsvp, the food and drug administration.
What is FSVP and how will it affect me? Safefood 360°
Web ( 1) appears on the current version of a list, issued by the food safety authority of the country in which the foreign supplier is located and which has regulatory oversight of the. Web as an importer, once you have completed foreign supplier verification programs (fsvp) for each of your food items from your foreign suppliers, you can file..
Foreign Supplier Verification Programs (FSVP) A Guide for Importers
Determining the fsvp importer ; Web if your facility is subject to fsvp, the food and drug administration (fda) has now released a document for foreign supplier verification programs for importers. Web policy regarding certain entities subject to the current good manufacturing practice and preventive controls, produce safety, and/or foreign supplier verification. Web fsvp foreign supplier evaluation form rating ★.
Form FDA483a Download Fillable PDF or Fill Online Fsvp Observations
Web approved foreign suppliers (with limited exceptions). Web policy regarding certain entities subject to the current good manufacturing practice and preventive controls, produce safety, and/or foreign supplier verification. Consumers, the foreign supplier verification programs (fsvp) developed a rule titled importers of food for human and animal. 384a (g)), we are publishing a list on our website of importers subject to..
FSPCA Foreign Supplier Verification Programs (FSVP) Course LiveVirtual
384a (g)), we are publishing a list on our website of importers subject to. Web policy regarding certain entities subject to the current good manufacturing practice and preventive controls, produce safety, and/or foreign supplier verification. Determining the fsvp importer ; Consumers, the foreign supplier verification programs (fsvp) developed a rule titled importers of food for human and animal. Web the.
U.S. FDA Foreign Supplier Verification Program (FSVP) Requirements
Food importers have required to verify. 384a (g)), we are publishing a list on our website of importers subject to. Web supplier, the default verification procedure is the performance of a properly conducted onsite audit of the foreign supplier before initially importing the food and at least. Web evaluate risks posed by the food and the performance of the foreign.
FSMA Foreign Supplier Verification Programs FSVP (Team Training
384a (g)), we are publishing a list on our website of importers subject to. Web this is commonly achieved by reviewing the foreign supplier's food safety records, auditing reports, and performance past. Implement corrective action(s), if needed. Food and drug administration (fda). Web ( 1) appears on the current version of a list, issued by the food safety authority of.
O The Hazard Analysis For The Food;
Web fsvp is a program that importers covered by the rule must have in place to verify that their foreign suppliers are producing food in a manner that provides the same level of public. Implement corrective action(s), if needed. O the entity that will be applying hazard controls, such. Consumers, the foreign supplier verification programs (fsvp) developed a rule titled importers of food for human and animal.
Web Evaluate Risks Posed By The Food And The Performance Of The Foreign Supplier, Considering:
Determine and apply appropriate verification activities and assess results. Web policy regarding certain entities subject to the current good manufacturing practice and preventive controls, produce safety, and/or foreign supplier verification. Web in accordance with section 805 (g) of the federal food, drug, and cosmetic act (21 u.s.c. Web the foreign suppliers verification programs (fsvp) importer portal for fsvp records submission is a means for importers to upload fsvp records.
Web Supplier, The Default Verification Procedure Is The Performance Of A Properly Conducted Onsite Audit Of The Foreign Supplier Before Initially Importing The Food And At Least.
Web the food and drug administration (fda) is adopting a regulation on foreign supplier verification programs (fsvps) for importers of food for humans and animals. Web fsvp foreign supplier evaluation form rating ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ 4.8 satisfied 20 votes how to fill out and sign fsvp program template online? Food and drug administration (fda). Determining the fsvp importer ;
Web Approved Foreign Suppliers (With Limited Exceptions).
Food importers have required to verify. Web if your facility is subject to fsvp, the food and drug administration (fda) has now released a document for foreign supplier verification programs for importers. Web for each entry line of food subject to the foreign supplier verification program (fsvp), offered for importation into the united states, you must ensure that. Web ( 1) appears on the current version of a list, issued by the food safety authority of the country in which the foreign supplier is located and which has regulatory oversight of the.