How Can Two Different Nonmetals Form A Compound
How Can Two Different Nonmetals Form A Compound - Web we destinguish four different type of compounds that might arise from two metals. Because if we combine 2 (or more) metals the resulting material doesn’t qualify as an a “compound”, we usually. Why do 2 metals not combine to form a compound? This has a clear stoichiometry (sodium thallide). How do nonmetals form bonds? Often, the nonmetal reactants can combine in different ratios and produce. Web explain why can two nonmetals bond together, but two metals cannot? A molecular compound is formed between two nonmetals (such as carbon dioxide). Two nonmetals, hydrogen and helium, make up about 99% of ordinary. Web metals often react with nonmetals to form ionic compounds.
Which nonmetals have similar chemical properties? This means that there is no definite. A molecular compound is formed between two nonmetals (such as carbon dioxide). Web a compound is a substance that contains two or more elements chemically combined in a fixed proportion. Why do 2 metals not combine to form a compound? How do nonmetals form bonds? Web nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds. The elements carbon and hydrogen combine to form many different. Web metals often react with nonmetals to form ionic compounds. Nonmetals have relatively high electronegativity, so both atoms in the bond want to keep the electrons that are being shared between them.
How do nonmetals form bonds? Web a compound is a substance that contains two or more elements chemically combined in a fixed proportion. This has a clear stoichiometry (sodium thallide). Web we destinguish four different type of compounds that might arise from two metals. Two nonmetals, hydrogen and helium, make up about 99% of ordinary. These compounds are composed of positive and negative ions formed by adding or subtracting electrons from. Why do 2 metals not combine to form a compound? Ionic compounds and molecular compounds. Generally, there are two types of inorganic compounds that can be formed: Web nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds.
Nonmetal Elements Science notes, Periodic table, Periodic table of
Web answer (1 of 7): Generally, there are two types of inorganic compounds that can be formed: Nonmetals have relatively high electronegativity, so both atoms in the bond want to keep the electrons that are being shared between them. Web although there definitely is such a thing as metallic bonding, when we combine two or more metals, the result is.
Reading The Periodic Table of Elements Biology (Early Release)
How do nonmetals form bonds? The elements carbon and hydrogen combine to form many different. Nonmetals have relatively high electronegativity, so both atoms in the bond want to keep the electrons that are being shared between them. A molecular compound is formed between two nonmetals (such as carbon dioxide). Binary ionic compounds containing a metal and a nonmetal 2.
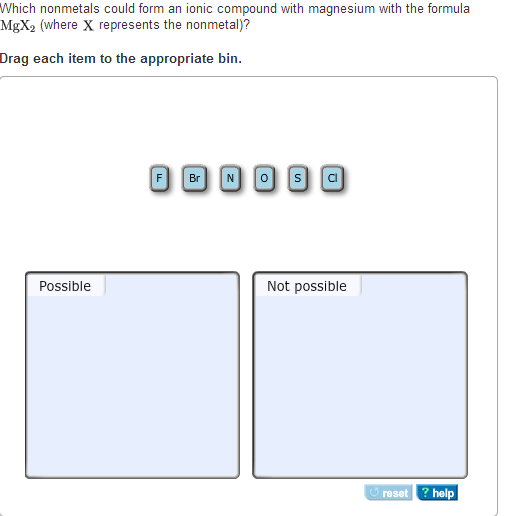
Solved Which nonmetals could form an ionic compound with
The elements carbon and hydrogen combine to form many different. A molecular compound is formed between two nonmetals (such as carbon dioxide). Which nonmetals have similar chemical properties? Web ionic compounds containing a metal and nomenclature of ionic and covalent compounds 1. Web we destinguish four different type of compounds that might arise from two metals.
CLILstore unit 2871 The classification of the elements
Web two nonmetals combine to form a covalent or molecular compound (i.e., one that is held together by covalent bonds which result from the sharing of electrons). Nonmetals have relatively high electronegativity, so both atoms in the bond want to keep the electrons that are being shared between them. Web we destinguish four different type of compounds that might arise.
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge
Often, the nonmetal reactants can combine in different ratios and produce. Binary ionic compounds containing a metal and a nonmetal 2. Web we destinguish four different type of compounds that might arise from two metals. A molecular compound is formed between two nonmetals (such as carbon dioxide). These compounds are composed of positive and negative ions formed by adding or.
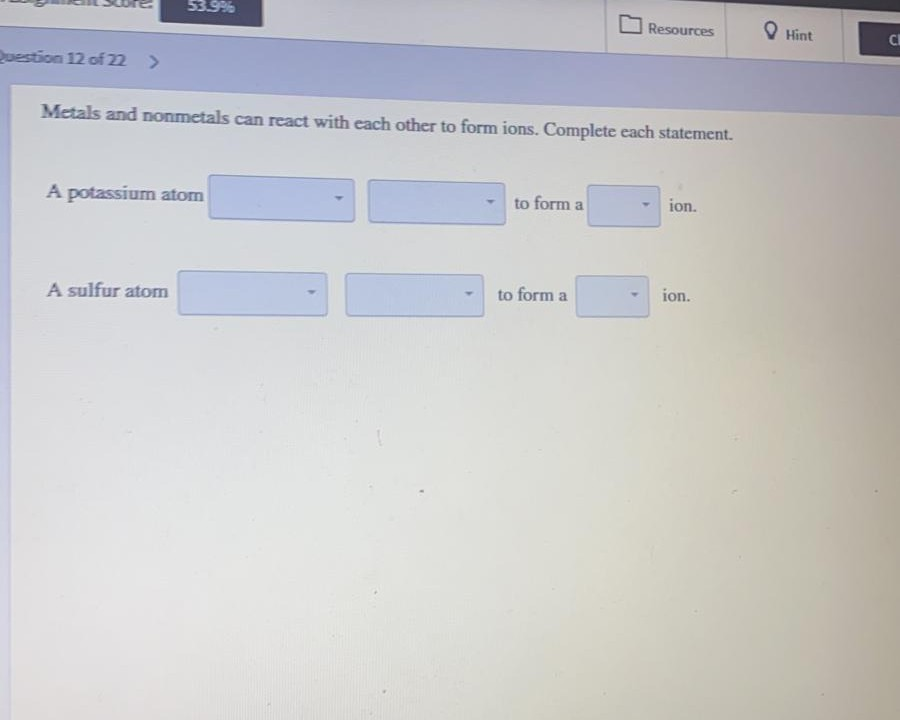
Solved 53.9 Resources Hint СІ Question 12 of 22 > Metals
A molecular compound is formed between two nonmetals (such as carbon dioxide). Web nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds. Ionic compounds and molecular compounds. Web answer (1 of 7): Web although there definitely is such a thing as metallic bonding, when we combine two or more metals, the result is a mixture.
The Parts of the Periodic Table
Which nonmetals have similar chemical properties? Web we destinguish four different type of compounds that might arise from two metals. Binary ionic compounds containing a metal and a nonmetal 2. Web metals often react with nonmetals to form ionic compounds. Why do 2 metals not combine to form a compound?
Covalent Compounds Examples and Properties
Web we destinguish four different type of compounds that might arise from two metals. The elements carbon and hydrogen combine to form many different. Web nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds. Why do 2 metals not combine to form a compound? Web explain why can two nonmetals bond together, but two metals.
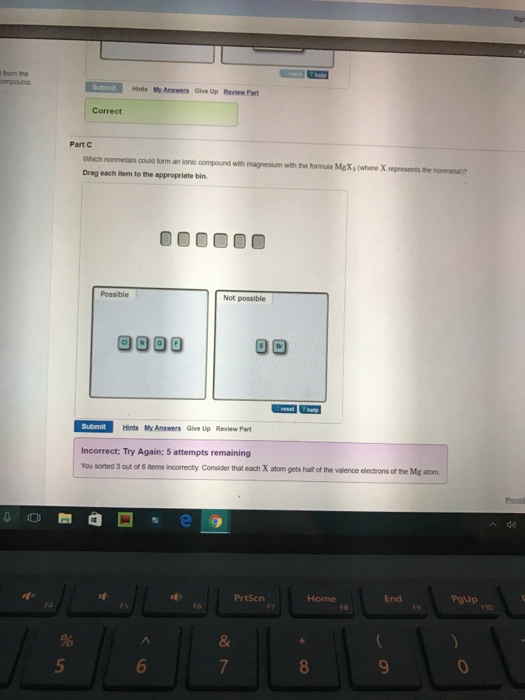
Solved Which nonmetals could form an ionic compound with
These compounds are composed of positive and negative ions formed by adding or subtracting electrons from. A molecular compound is formed between two nonmetals (such as carbon dioxide). The elements carbon and hydrogen combine to form many different. Web a compound is a substance that contains two or more elements chemically combined in a fixed proportion. Generally, there are two.
Metals vs Nonmetals
Which nonmetals have similar chemical properties? Two nonmetals, hydrogen and helium, make up about 99% of ordinary. Why do 2 metals not combine to form a compound? Web we destinguish four different type of compounds that might arise from two metals. Web nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds.
Generally, There Are Two Types Of Inorganic Compounds That Can Be Formed:
A molecular compound is formed between two nonmetals (such as carbon dioxide). Web when nonmetals react with one another, the product is a molecular compound. Web explain why can two nonmetals bond together, but two metals cannot? Web metals often react with nonmetals to form ionic compounds.
This Means That There Is No Definite.
Web a compound is a substance that contains two or more elements chemically combined in a fixed proportion. This has a clear stoichiometry (sodium thallide). Binary ionic compounds containing a metal and a nonmetal 2. Because if we combine 2 (or more) metals the resulting material doesn’t qualify as an a “compound”, we usually.
Often, The Nonmetal Reactants Can Combine In Different Ratios And Produce.
Web two nonmetals combine to form a covalent or molecular compound (i.e., one that is held together by covalent bonds which result from the sharing of electrons). Web answer (1 of 7): Two nonmetals, hydrogen and helium, make up about 99% of ordinary. How do nonmetals form bonds?
Why Do 2 Metals Not Combine To Form A Compound?
Which nonmetals have similar chemical properties? Nonmetals have relatively high electronegativity, so both atoms in the bond want to keep the electrons that are being shared between them. Web ionic compounds containing a metal and nomenclature of ionic and covalent compounds 1. The elements carbon and hydrogen combine to form many different.