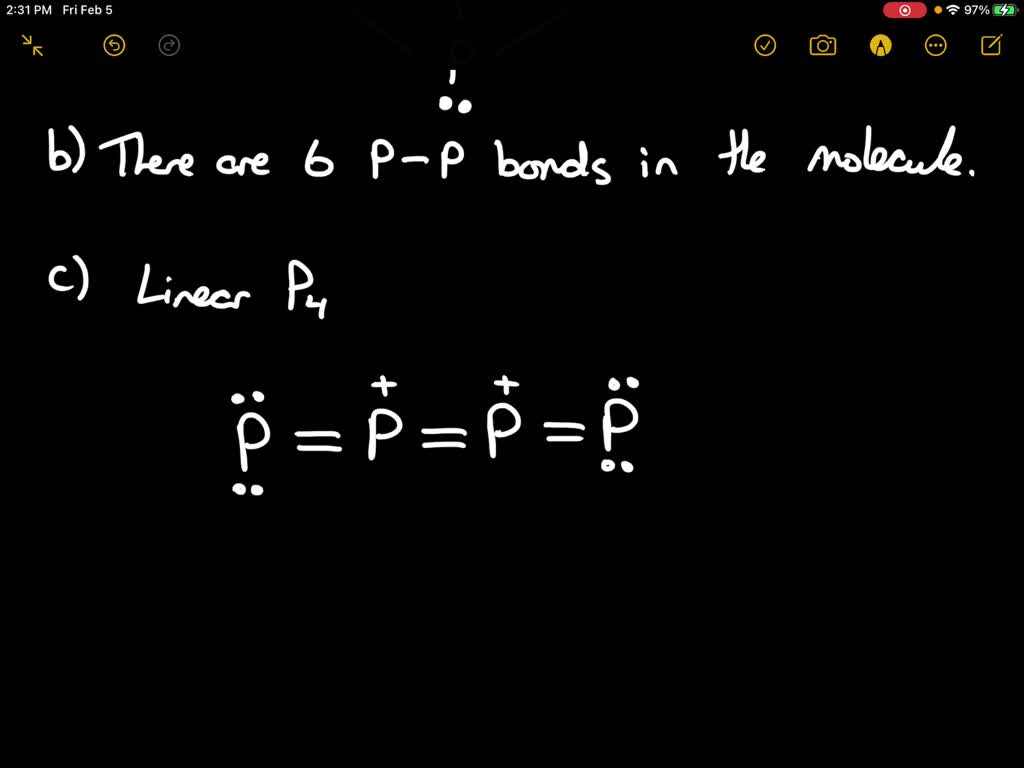How Many Bonds Does Phosphorus Form
How Many Bonds Does Phosphorus Form - Web the reason that phosphorus can form “five bonds” and nitrogen only three or four has to do with the size of the two atoms. Because the compound does not contain mostly carbon and hydrogen, it is inorganic. Phosphorus only 'needs' three more electrons to get a full valence shell of eight, but you'll notice that it actually has five valence electrons, so in. Fluorine and the other halogens in group 7a (17) have seven valence. For more information on the visual elements. Web because phosphorus does not form strong multiple bonds with itself, elemental phosphorus consists of tetrahedral p 4 molecules in which each atom forms single. Phosphorous has 5 valence electrons like nitrogen and has a good chance of forming 3 bonds with one lone pair to make an octet. Web based on the lewis dot diagram for phosphorus, how many bonds does phosphorous prefer to form in a covalent molecule? Hence, sulphur only forms rings and chains. How many bonds can phosphorus make, and explain how this number was found?
For more information on the visual elements. Web based on the lewis dot diagram for phosphorus, how many bonds does phosphorous prefer to form in a covalent molecule? Web since it can form three bonds, phosphorus can form a p4 white phosphorus tetrahedron, while sulphur can only form two bonds. How many bonds can phosphorus make, and explain how this number was found? Web how many bonds does phosphorus typically make? But phosphorous has empty d. Web the molecule has 4 phosphorus atoms and 3 sulfur atoms. Web allotropes some elements exist in several different structural forms, called allotropes. Web how many bonds does the phosphorus atom form? Web the reason that phosphorus can form “five bonds” and nitrogen only three or four has to do with the size of the two atoms.
Luckily, every phosphorus atom is looking to gain three electrons. Web aluminum (al) and phosphorus (p) can also bond. Web phosphorus (p), nonmetallic chemical element of the nitrogen family (group 15 [va] of the periodic table) that at room temperature is a colourless, semitransparent, soft, waxy solid. Fluorine and the other halogens in group 7a (17) have seven valence. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Because the compound does not contain mostly carbon and hydrogen, it is inorganic. Web how many bonds can phosphorus make? Web how many bonds does phosphorus typically make? Web since it can form three bonds, phosphorus can form a p4 white phosphorus tetrahedron, while sulphur can only form two bonds. The α form is defined as the standard state of the element, but is actually.
How Many Electrons Are There In A Fluoride Ion pauloqdesigner
How many bonds can phosphorus make, and explain how this number was found? Web because phosphorus does not form strong multiple bonds with itself, elemental phosphorus consists of tetrahedral p 4 molecules in which each atom forms single. Phosphorous has 5 valence electrons like nitrogen and has a good chance of forming 3 bonds with one lone pair to make.
Phosphorus foods, functions, how much do you need & more Eufic
Web the reason that phosphorus can form “five bonds” and nitrogen only three or four has to do with the size of the two atoms. Web phosphorus (p), nonmetallic chemical element of the nitrogen family (group 15 [va] of the periodic table) that at room temperature is a colourless, semitransparent, soft, waxy solid. Aluminum happens to have three extra electrons..
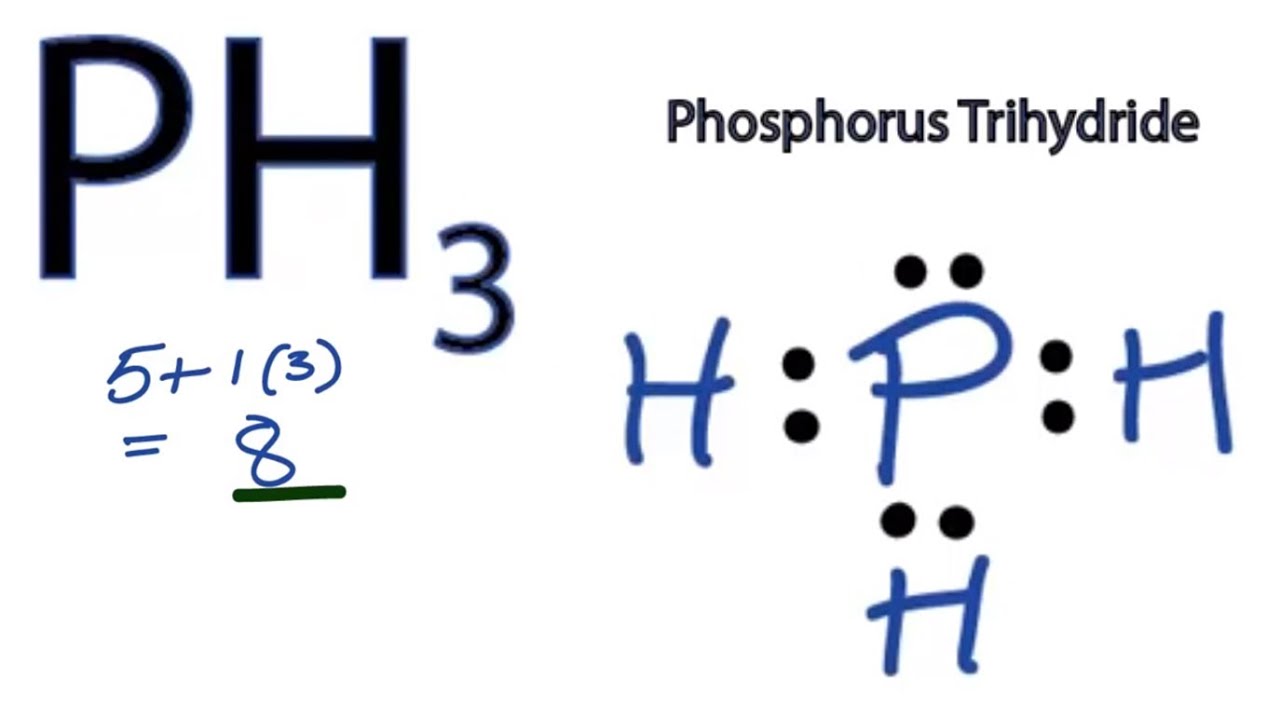
Lewis Dot Diagram Phosphorus
But phosphorous has empty d. Two crystalline forms are known. Web how many bonds can phosphorus make? Phosphorous has 5 valence electrons like nitrogen and has a good chance of forming 3 bonds with one lone pair to make an octet. How many electrons surround the phosphorous atom?
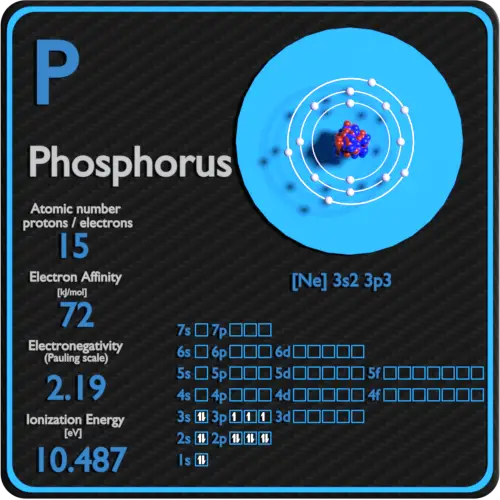
Phosphorus Periodic Table and Atomic Properties
Two crystalline forms are known. Web phosphorus (p), nonmetallic chemical element of the nitrogen family (group 15 [va] of the periodic table) that at room temperature is a colourless, semitransparent, soft, waxy solid. Web since it can form three bonds, phosphorus can form a p4 white phosphorus tetrahedron, while sulphur can only form two bonds. Web aluminum (al) and phosphorus.
LabXchange
Web based on the lewis dot diagram for phosphorus, how many bonds does phosphorous prefer to form in a covalent molecule? Two crystalline forms are known. For more information on the visual elements. Web the molecule has 4 phosphorus atoms and 3 sulfur atoms. Fluorine and the other halogens in group 7a (17) have seven valence.
Phosphorus Cycle Wetland Science and Practice Pinterest
Web based on the lewis dot diagram for phosphorus, how many bonds does phosphorous prefer to form in a covalent molecule? For more information on the visual elements. Web aluminum (al) and phosphorus (p) can also bond. Luckily, every phosphorus atom is looking to gain three electrons. How many lone pairs are around the phosphorous atom?
How does phosphorus form 5 covalent bonds? The Unconditional Guru
Web since it can form three bonds, phosphorus can form a p4 white phosphorus tetrahedron, while sulphur can only form two bonds. Web phosphorus #s^2 p^3# has a 5 valence electrons and needs three more to complete the rule of octet. The α form is defined as the standard state of the element, but is actually. Web answer (1 of.
SOLVEDA common form of elemental phosphorus is the tetrahedral \mathrm
Phosphorous has 5 valence electrons like nitrogen and has a good chance of forming 3 bonds with one lone pair to make an octet. Aluminum happens to have three extra electrons. Web the reason that phosphorus can form “five bonds” and nitrogen only three or four has to do with the size of the two atoms. Fluorine and the other.
Periodic Table Phosphorus Valence Electrons Periodic Table Timeline
Two crystalline forms are known. Web based on the lewis dot diagram for phosphorus, how many bonds does phosphorous prefer to form in a covalent molecule? How many lone pairs are around the phosphorous atom? Web since it can form three bonds, phosphorus can form a p4 white phosphorus tetrahedron, while sulphur can only form two bonds. How many bonds.
FileElectron shell 015 Phosphorus.svg Wikimedia Commons Phosphorus
Web phosphorus #s^2 p^3# has a 5 valence electrons and needs three more to complete the rule of octet. Phosphorus only 'needs' three more electrons to get a full valence shell of eight, but you'll notice that it actually has five valence electrons, so in. Two crystalline forms are known. Luckily, every phosphorus atom is looking to gain three electrons..
Web Phosphorus (P), Nonmetallic Chemical Element Of The Nitrogen Family (Group 15 [Va] Of The Periodic Table) That At Room Temperature Is A Colourless, Semitransparent, Soft, Waxy Solid.
But phosphorous has empty d. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Web based on the lewis dot diagram for phosphorus, how many bonds does phosphorous prefer to form in a covalent molecule?
Hence, Sulphur Only Forms Rings And Chains.
Chlorine #s^2 p^5# has 7 valence electrons and needs one to complete the. Covalent bond two atoms form a covalent chemical bond by each sharing at least one of their available valence electrons. Web how many bonds does the phosphorus atom form? Fluorine and the other halogens in group 7a (17) have seven valence.
Two Crystalline Forms Are Known.
Phosphorus only 'needs' three more electrons to get a full valence shell of eight, but you'll notice that it actually has five valence electrons, so in. Web how many bonds does phosphorus typically make? Web the reason that phosphorus can form “five bonds” and nitrogen only three or four has to do with the size of the two atoms. Web aluminum (al) and phosphorus (p) can also bond.
How Many Lone Pairs Are Around The Phosphorous Atom?
Because the compound does not contain mostly carbon and hydrogen, it is inorganic. Web phosphorus #s^2 p^3# has a 5 valence electrons and needs three more to complete the rule of octet. Web answer (1 of 3): Web since it can form three bonds, phosphorus can form a p4 white phosphorus tetrahedron, while sulphur can only form two bonds.