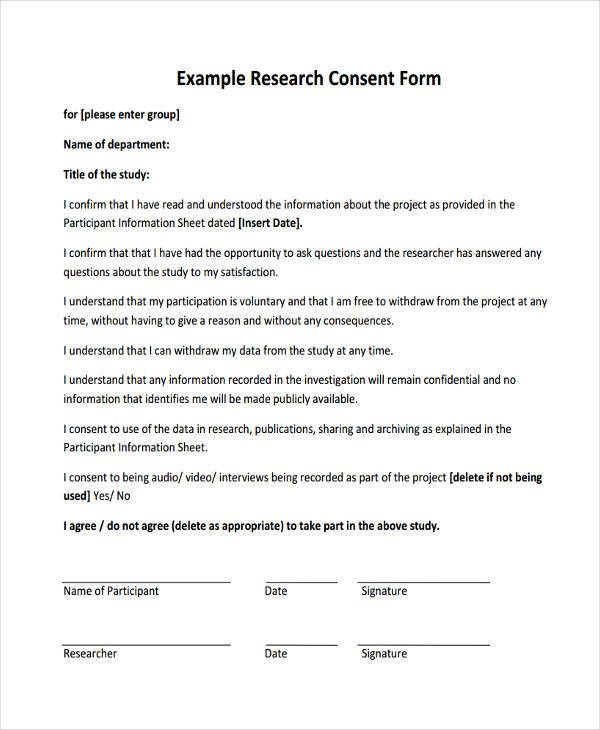
Sample Consent Form For Research Survey
Sample Consent Form For Research Survey - If an online survey is to be conducted, the investigator must submit a printed copy of the consent form and data instrument as it will appear in the final format that is presented to participants. When completing and irb submission in irbis, please fill in the application and use the consent form builder specific to your project. The consent form (icf) templates provided by the irb comply with federal regulations and hipaa. Web consent form templates. The form explains to participants what the survey is about, what their participation will entail, and what their rights are. Parental permission and child assent form templates Time to complete 2 minutes eligibility It aims at identifying best teaching practices by understanding practices and beliefs of. Am asking you to participate in my research study. Web clicking a “submit” button.
What is the study about? The contents can easily be modified and fields can easily be added. Am asking you to participate in my research study. Customize this template to reflect the specifics of your study and participant population. You are invited to participate in a study being conducted by dr. Name department address city state phone number. Include the project title on all pages of the consent form. It aims at identifying best teaching practices by understanding practices and beliefs of. Please use the microsoft readability statistics tool as needed when writing your consent form. The form explains to participants what the survey is about, what their participation will entail, and what their rights are.
This is usually more complex than just answering a series of questions. Web defines the term informed consent process and provides tips and other information to craft an appropriate informed consent document for a human subjects study and univeristy of michigan irb review. Standard adult informed consent form. This is a consent form for participants for a research study. This is if the survey just involves answering a questionnaire. The consent form (icf) templates provided by the irb comply with federal regulations and hipaa. Web click here for guidance on informed consent from the office of human research protection (ohrp) general consent form templates. The goal of this research study is to… You must modify this form to ensure that it is applicable to your study. Includes links to informed consent templates and.
FREE 6+ Sample Survey Consent Forms in PDF MS Word
Web clicking a “submit” button. Delete all colored text from the final copy of your form. It aims at identifying best teaching practices by understanding practices and beliefs of. The form explains to participants what the survey is about, what their participation will entail, and what their rights are. Web the title of protocol must match the title on all.
FREE 6+ Sample Survey Consent Forms in PDF MS Word
Includes links to informed consent templates and. The form clearly states the details and purpose of the project, as well as why the particular topic has been chosen for the exercise. The title must be relevant, appropriate, and easy to understand. Web obtain consent efficiently and conveniently from your research participants by using this research consent form. Web he below.
FREE 31+ Survey Forms in PDF Excel MS word
The contents can easily be modified and fields can easily be added. The form explains to participants what the survey is about, what their participation will entail, and what their rights are. Include the project title on all pages of the consent form. This is if the survey just involves answering a questionnaire. Web clicking a “submit” button.
Sample Survey Consent Form 9+ Free Documents Download in PDF, Word
I am a graduate student/faculty member in the department/school/college of ________________ at southern illinois university carbondale. You must modify this form to ensure that it is applicable to your study. Standard adult informed consent form. Web sample informed consent for online interview research consent for participation in the study the researcher requests your consent for participation in a study about.
FREE 8+ Research Consent Forms in PDF MS Word
For more information, please find instructions here. Web sample survey consent form my name is ___________. It aims at identifying best teaching practices by understanding practices and beliefs of. Parental permission and child assent form templates Web the title of protocol must match the title on all consent forms.
FREE 6+ Research Consent Forms in PDF MS Word
Web sample consent form for online surveys modify to fit your study. This consent form asks you to allow the researcher to record and view the interview and to use your comments to enhance understanding of the topic. Web consent to act as a participant project title: You must modify this form to ensure that it is applicable to your.
FREE 6+ Sample Survey Consent Forms in PDF MS Word
General consent form templates social and behavioral research projects (last updated 03/16/2023) For more information, please find instructions here. This form template is free and can be customized easily using the form builder. When completing and irb submission in irbis, please fill in the application and use the consent form builder specific to your project. You are invited to participate.
FREE 7+ Survey Consent Forms in PDF MS Word
When completing and irb submission in irbis, please fill in the application and use the consent form builder specific to your project. Web the title of protocol must match the title on all consent forms. Web click here for guidance on informed consent from the office of human research protection (ohrp) general consent form templates. Web a survey consent form.
FREE 8+ Sample Survey Consent Forms in PDF MS Word
Web survey research consent form: Web this survey consent form template is one simple and clean survey consent form that anyone can use. A backslash (e.g., will/will not) requires a selection depending. Includes links to informed consent templates and. This is if the survey just involves answering a questionnaire.
FREE 8+ Sample Research Consent Forms in PDF MS Word
Web defines the term informed consent process and provides tips and other information to craft an appropriate informed consent document for a human subjects study and univeristy of michigan irb review. These consent form templates have been posted for your reference. Time to complete 2 minutes eligibility Web in the case of scientific survey exercises, a consent research survey form.
Jane Doe, A Unc Charlotte Associate Professor In The Department Of Psychology.
Am asking you to participate in my research study. Web survey research consent form: A backslash (e.g., will/will not) requires a selection depending. What is the study about?
This Consent Form Asks You To Allow The Researcher To Record And View The Interview And To Use Your Comments To Enhance Understanding Of The Topic.
This is a research project. Web a survey consent form is a form that is used by researchers when conducting a survey. You must modify this form to ensure that it is applicable to your study. Parental permission and child assent form templates
The Purpose Of My Study Is To________.
It aims at identifying best teaching practices by understanding practices and beliefs of. Time to complete 2 minutes eligibility It also obtains the participant's permission to use their information for research purposes. Web defines the term informed consent process and provides tips and other information to craft an appropriate informed consent document for a human subjects study and univeristy of michigan irb review.
If An Online Survey Is To Be Conducted, The Investigator Must Submit A Printed Copy Of The Consent Form And Data Instrument As It Will Appear In The Final Format That Is Presented To Participants.
You are invited to participate in a study being conducted by dr. Title of your study informed consent you are invited to participate in a research study about. The title must be relevant, appropriate, and easy to understand. The contents can easily be modified and fields can easily be added.