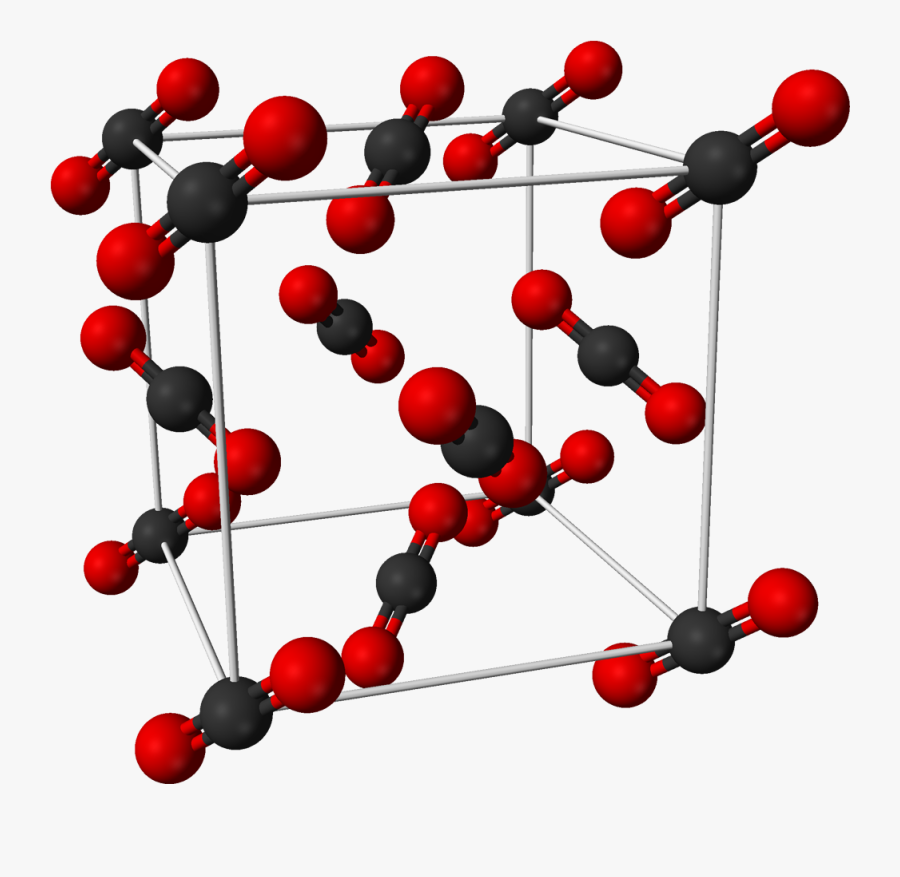
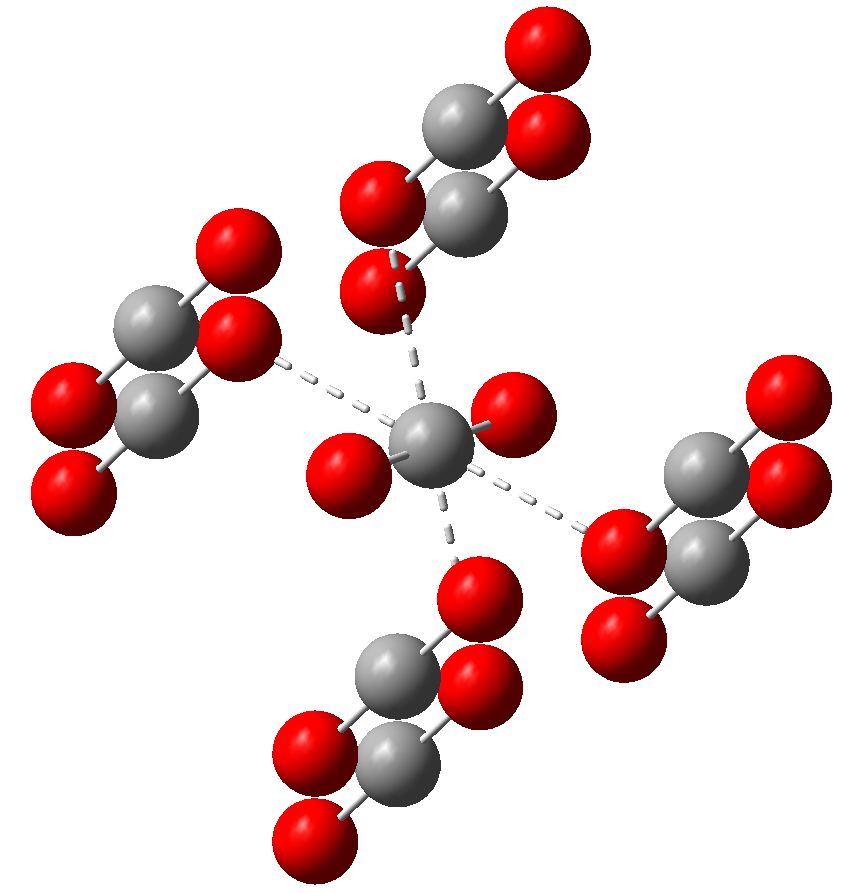
Solid Form Of Carbon Dioxide
Solid Form Of Carbon Dioxide - Ou chemical safety data (no longer updated) more details −109.3 °f) at earth atmospheric pressure — that is, it transitions directly from solid to. At room temperature and pressure, it sublimates into carbon dioxide vapor. The common name of solid carbon dioxide (co2) is “dry ice”. In this reaction, solid carbon latches onto one of the oxygen atoms in carbon dioxide gas,. Co2) is a colorless gas. In typical co 2 capture processes, the co 2 gas is pressurised to a liquid, which is then transported to a. They are widely distributed all over the world. Solid form is utilized as dry ice.] Web carbon dioxide, co2, is a colourless and odorless gas.
Solid carbon dioxide is always below −78.5 °c (−109.3 °f) at regular atmospheric pressure, regardless of the air temperature. They are currently the largest energy sources for electricity production in conventional thermal power plants. Co2) is a colorless gas. Web answer (1 of 10): The chemical structure is centrosymmetric and linear, so carbon dioxide has no electric dipole. It is formed by combustion and by biological processes. Web in solid form, it is called dry ice. Web solid carbons such as coal and biomass are cheap fuels with high energy density in volume. At room temperature and pressure, it sublimates into carbon dioxide vapor. People and animals release carbon dioxide when they breathe out.
Solid carbon dioxide is always below −78.5 °c (−109.3 °f) at regular atmospheric pressure, regardless of the air temperature. Web carbon dioxide (chemical formula: Ou chemical safety data (no longer updated) more details In this reaction, solid carbon latches onto one of the oxygen atoms in carbon dioxide gas,. It is also used in laboratories for reacting chemicals that require very low temperature. Solid form is utilized as dry ice.] Web solid carbons such as coal and biomass are cheap fuels with high energy density in volume. Freezer burn results from the sublimation of ice into water vapor. Web in solid form, it is called dry ice. At the right temperature, the elements iodine and arsenic will sublimate from solid form into gaseous form.
A view to a kill in the morning Carbon dioxide Scientific American
Solid co 2 sublimes at 194.65 k (−78.5 °c; −109.3 °f) at earth atmospheric pressure — that is, it transitions directly from solid to. Freezer burn results from the sublimation of ice into water vapor. Web answer (1 of 10): They are currently the largest energy sources for electricity production in conventional thermal power plants.
File Carbon Dioxide Unit Solid Carbon Dioxide Structure , Free
Suggest new co2 full form. It is relatively nontoxic and noncombustible, but it is heavier than air and may asphyxiate by the displacement of air. It is absorbed by plants in photosynthesis. Web carbonic acid gas, dry ice [note: Web dry ice is the solid form of carbon dioxide (co 2), a molecule consisting of a single carbon atom bonded.
Media Portfolio
In typical co 2 capture processes, the co 2 gas is pressurised to a liquid, which is then transported to a. Normal constituent of air (about 300 ppm)]. Web science news from research organizations decarbonization tech instantly converts carbon dioxide to solid carbon date: Web carbon dioxide, (co 2), a colourless gas having a faint sharp odour and a sour.
Illustrated Glossary of Organic Chemistry Carbon dioxide
Web carbon dioxide (chemical formula: People and animals release carbon dioxide when they breathe out. Web carbon dioxide forms a weak acid, called carbonic acid (h 2 co 3), when dissolved in water. Solid co 2 sublimes at 194.65 k (−78.5 °c; Web answer (1 of 6):
There is More Carbon Dioxide in the Atmosphere Than Ever Before
At the right temperature, the elements iodine and arsenic will sublimate from solid form into gaseous form. They are currently the largest energy sources for electricity production in conventional thermal power plants. Web sublimation examples dry ice is solid carbon dioxide. Web dry ice is the solid form of carbon dioxide, a molecule that is found as a gas in.
What is the solid form of carbon... Trivia Questions
This is a very used and efficient refrigerant, which when losing cold does not transform into water (like common ice) but turns into gas (since at atmospheric pressure it is gaseous), which tends to minimize microbial contamination in fresh. Web solid carbon dioxide is called dry ice and is used for small shipments where refrigeration equipment is not practical. The.
Researchers discover a way to tease oxygen molecules from carbon dioxide
Web carbon dioxide (chemical formula: Web carbon dioxide forms a weak acid, called carbonic acid (h 2 co 3), when dissolved in water. Solid, frozen carbon dioxide is called dry ice. −109.3 °f) at earth atmospheric pressure — that is, it transitions directly from solid to. Carbon dioxide is a gas at room temperature,.
How Much does Dry Ice Cost
Web carbon dioxide (chemical formula: Suggest new co2 full form. It is composed of one carbon ( c) and two oxygen ( o) atoms. Find out about the process of sublimation, the uses. Carbon dioxide is a gas at room temperature,.
Carbon dioxide wikidoc
Carbon dioxide is the most abundant gas in the atmospheres of mars and venus. Web carbon dioxide, solid chemical identifiers | hazards | response recommendations | physical properties | regulatory information | alternate chemical names chemical identifiers When co 2 is solved in water, the mild carbonic acid, is formed. Web dry ice is the solid form of carbon dioxide.
CO2solid
It is relatively nontoxic and noncombustible, but it is heavier than air and may asphyxiate by the displacement of air. Web carbon dioxide, (co 2), a colourless gas having a faint sharp odour and a sour taste. The common name of solid carbon dioxide (co2) is “dry ice”. In this reaction, solid carbon latches onto one of the oxygen atoms.
It Is Composed Of One Carbon ( C) And Two Oxygen ( O) Atoms.
Web carbonic acid gas, dry ice [note: Find out about the process of sublimation, the uses. At the right temperature, the elements iodine and arsenic will sublimate from solid form into gaseous form. The common name of solid carbon dioxide (co2) is “dry ice”.
The Chemical Or Molecular Formula For Carbon Dioxide Is Co 2.
Web in solid form, it is called dry ice. Cooled co 2 in solid form is called dry ice. Solid form is utilized as dry ice.] Web a new carbon capture process has been developed that turns carbon dioxide gas into solid carbon that is easier to store.
This Is A Very Used And Efficient Refrigerant, Which When Losing Cold Does Not Transform Into Water (Like Common Ice) But Turns Into Gas (Since At Atmospheric Pressure It Is Gaseous), Which Tends To Minimize Microbial Contamination In Fresh.
People and animals release carbon dioxide when they breathe out. Web carbon, either elemental or combined, is usually determined quantitatively by conversion to carbon dioxide gas, which can then be absorbed by other chemicals to give either a weighable product or a solution with acidic properties that can be titrated. Web solid carbons such as coal and biomass are cheap fuels with high energy density in volume. The chemical structure is centrosymmetric and linear, so carbon dioxide has no electric dipole.
Web Carbon Dioxide In Its Solid Form (Which Is Achieved By Exposure To Low Temperatures) Forms Dry Ice.
Carbon dioxide is the most abundant gas in the atmospheres of mars and venus. Freezer burn results from the sublimation of ice into water vapor. When a chunk of dry ice is exposed to room temperature air it undergoes sublimation, which means it changes from a solid directly into a gas, without melting into a liquid first. Dry ice is the solid form of carbon dioxide.