What Element Can Form The Most Covalent Bonds
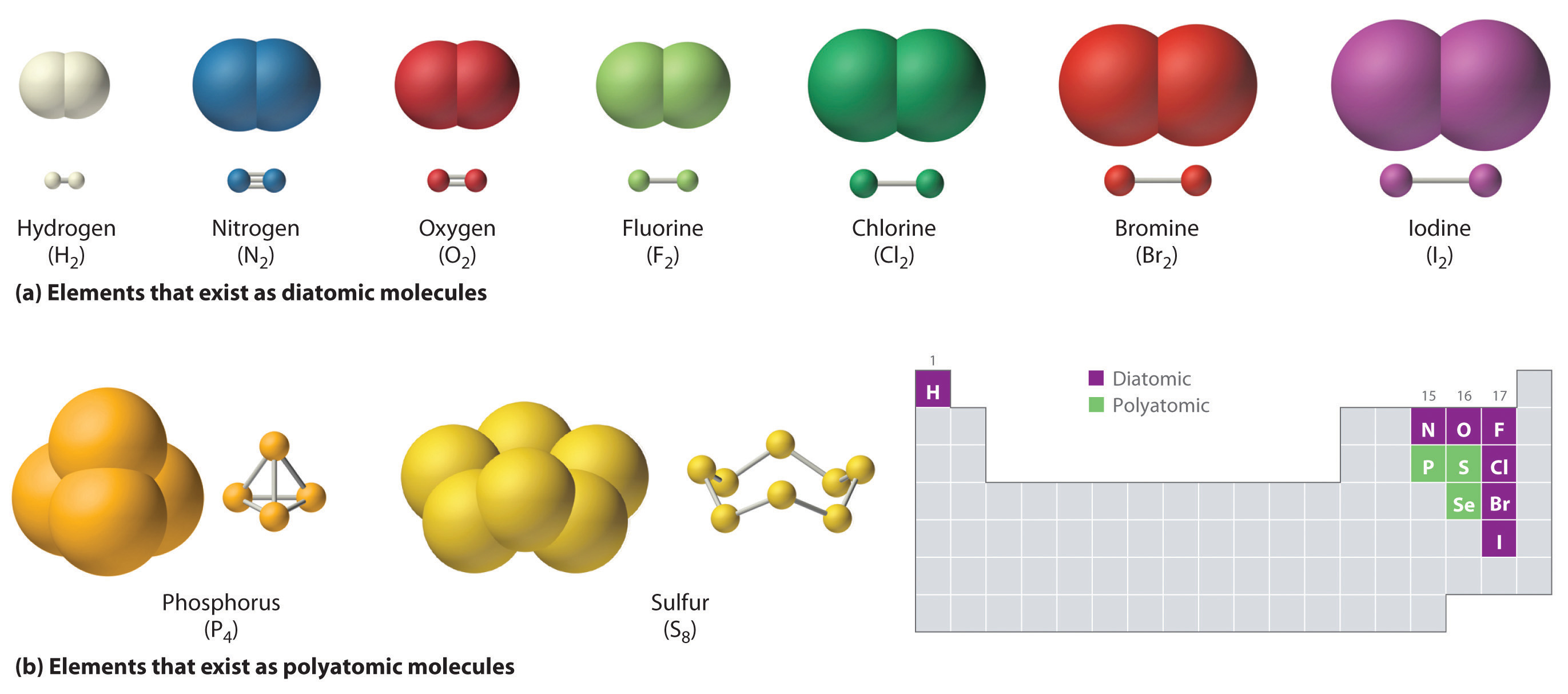
What Element Can Form The Most Covalent Bonds - A table of lewis dot symbols of nonmetal elements that form covalent bonds is shown in fig. Atoms can combine to achieve an octet of valence electrons by sharing electrons. So, what are covalent bond types of elements examples ? Web a covalent bond is formed when two atoms share a pair of electrons. Web covalent bonding covalent bonding is the sharing of electrons between atoms. I don’t know if metalloids also do that or not, though. Web silicones are polymeric compounds containing, among others, the following types of covalent bonds: Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another. Scientists can manipulate ionic properties and these interactions in. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds.
Two fluorine atoms, for example, can form a stable f 2 molecule in. Web covalent bond types of elements are wherein elements form bond by covalent bonding. When bond is formed by. Web ionic and covalent bonds are strong bonds that require considerable energy to break. Atoms can combine to achieve an octet of valence electrons by sharing electrons. Web covalent bonding covalent bonding is the sharing of electrons between atoms. Using the electronegativity values in. When none of the elements in a compound is a metal, no atoms in the compound have an ionization. Web a covalent bond is formed when two atoms share a pair of electrons. This type of bonding occurs between two atoms of the same element or of elements close to each.
Using the electronegativity values in. I don’t know if metalloids also do that or not, though. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Two fluorine atoms, for example, can form a stable f 2 molecule in. A table of lewis dot symbols of nonmetal elements that form covalent bonds is shown in fig. Web lewis dot structures are one way to represent how atoms form covalent bonds. Web a covalent bond is formed when two atoms share a pair of electrons. So, what are covalent bond types of elements examples ? In the diagram of methane shown here, the carbon atom has a valence of. Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another.
How to Predict number of bonds each element forms ChemSimplified
So, what are covalent bond types of elements examples ? Web lewis proposed that an atom forms enough covalent bonds to form a full (or closed) outer electron shell. Web covalent bond types of elements are wherein elements form bond by covalent bonding. Web answer (1 of 25): Web ionic and covalent bonds are strong bonds that require considerable energy.
Bonding A Level Notes
Web lewis proposed that an atom forms enough covalent bonds to form a full (or closed) outer electron shell. Web answer (1 of 25): The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight. Web covalent bonding covalent bonding is the sharing of electrons between atoms..
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Web ionic bonds are important because they allow the synthesis of specific organic compounds. Web lewis dot structures are one way to represent how atoms form covalent bonds. A table of lewis dot symbols of nonmetal elements that form covalent bonds is shown in fig. Web covalent bonding covalent bonding is the sharing of electrons between atoms. Web moreover, of.
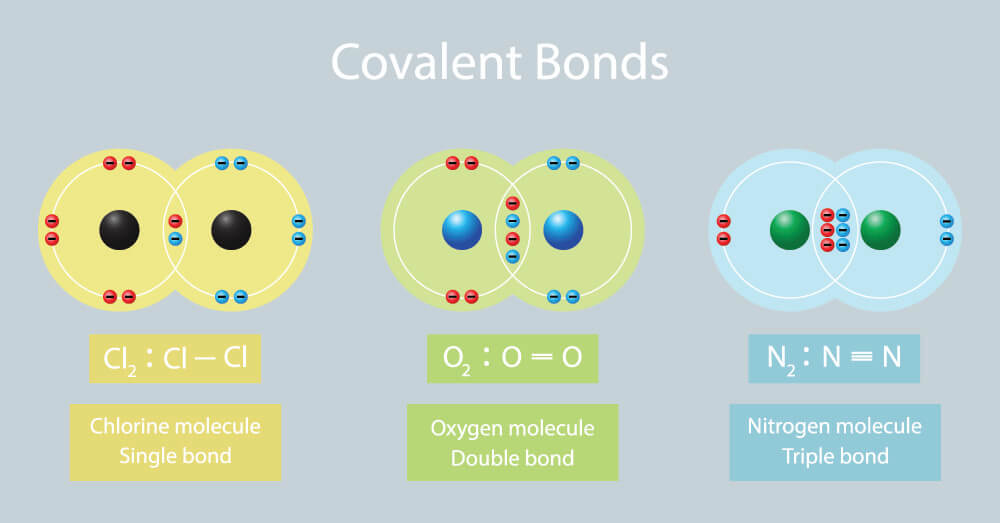
Single, Double, and Triple Bonds
Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. When none of the elements in a compound is a metal, no atoms in the compound have an ionization. Web ionic and covalent bonds are strong bonds that require considerable energy to break. Web answer.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Web how many covalent bonds are formed? So, what are covalent bond types of elements examples ? In the diagram of methane shown here, the carbon atom has a valence of. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Two fluorine atoms, for.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Scientists can manipulate ionic properties and these interactions in. This type of bonding occurs between two atoms of the same element or of elements close to each. Web answer (1 of 25): Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another. Web.
Covalent Bond Biology Dictionary
Web ionic bonds are important because they allow the synthesis of specific organic compounds. Scientists can manipulate ionic properties and these interactions in. When bond is formed by. Web a covalent bond is formed when two atoms share a pair of electrons. Two fluorine atoms, for example, can form a stable f 2 molecule in.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
When bond is formed by. Web lewis proposed that an atom forms enough covalent bonds to form a full (or closed) outer electron shell. Web how many covalent bonds are formed? Atoms can combine to achieve an octet of valence electrons by sharing electrons. However, not all bonds between elements are ionic or covalent bonds.
Covalent Bonds Biology for NonMajors I
In the diagram of methane shown here, the carbon atom has a valence of. Web ionic bonds are important because they allow the synthesis of specific organic compounds. So, what are covalent bond types of elements examples ? Atoms can combine to achieve an octet of valence electrons by sharing electrons. Using the electronegativity values in.
Covalent Bonding (Biology) — Definition & Role Expii
Web answer (1 of 25): Web covalent bonding covalent bonding is the sharing of electrons between atoms. Using the electronegativity values in. When bond is formed by. A table of lewis dot symbols of nonmetal elements that form covalent bonds is shown in fig.
Web Silicones Are Polymeric Compounds Containing, Among Others, The Following Types Of Covalent Bonds:
However, not all bonds between elements are ionic or covalent bonds. Web a covalent bond is formed when two atoms share a pair of electrons. Using the electronegativity values in. Web covalent bond types of elements are wherein elements form bond by covalent bonding.
When Bond Is Formed By.
Web how many covalent bonds are formed? Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Group iiib, ivb, vb, vib, viib, viiib,. Web lewis dot structures are one way to represent how atoms form covalent bonds.
The Number Of Bonds That An Atom Can Form Can Often Be Predicted From The Number Of Electrons Needed To Reach An Octet (Eight.
A table of lewis dot symbols of nonmetal elements that form covalent bonds is shown in fig. Web ionic and covalent bonds are strong bonds that require considerable energy to break. Scientists can manipulate ionic properties and these interactions in. Atoms can combine to achieve an octet of valence electrons by sharing electrons.
I Don’t Know If Metalloids Also Do That Or Not, Though.
This type of bonding occurs between two atoms of the same element or of elements close to each. Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another. Two fluorine atoms, for example, can form a stable f 2 molecule in. Web ionic bonds are important because they allow the synthesis of specific organic compounds.