What Is Required For Reactants To Form Bonds
What Is Required For Reactants To Form Bonds - Energy is required to break bonds. The reactants must have the correct orientation and geometry to allow for bonding. Web when a chemical reaction occurs, molecular bonds are broken and other bonds are formed to make different molecules. Web what is required for reactants to form bonds? Web in order for reactants to form bonds, three conditions need to be satisfied: For example, the bonds of two water. Web bond making is generally an endothermic reaction so input energy would be required to make a reactants bond. A compound partitions into its components. This means that the atoms or molecules must be positioned in a way that allows for the. Energy output energy input equilibrium exchange reaction answers answer 1 energy input is required for reactants to form.
The reactants must have the correct orientation and geometry to allow for bonding. As a result, the surroundings get cold. Energy is required to break bonds. The reactant molecules must collide. Web what is required for reactants to form bonds? Web heat is absorbed by reactants to form products. Heat is absorbed from the surroundings; Web the reactants must have the correct chemical properties to allow for bond formation. Energy is released when chemical bonds are. Web atoms are held together by a certain amount of energy called bond energy.
Web in order for reactants to form bonds, three conditions need to be satisfied: As a result, the surroundings get cold. Web what is required for reactants to form bonds? Web what is required for reactants to form bonds? The reactants must have the correct orientation and geometry to allow for bonding. This means that the atoms or molecules must be positioned in a way that allows for the. Web energy is released when new bonds form. Web what is required for reactants to form bonds? Web when a chemical reaction occurs, molecular bonds are broken and other bonds are formed to make different molecules. Web bond making is generally an endothermic reaction so input energy would be required to make a reactants bond.
It's important to form bonds. dynastywarriors
The ribosome serves as the site and carries the. Web what is required for reactants to form bonds? Web what is required for reactants to form bonds? Web in order for reactants to form bonds, three conditions need to be satisfied: Energy output energy input equilibrium exchange reaction answers answer 1 energy input is required for reactants to form.
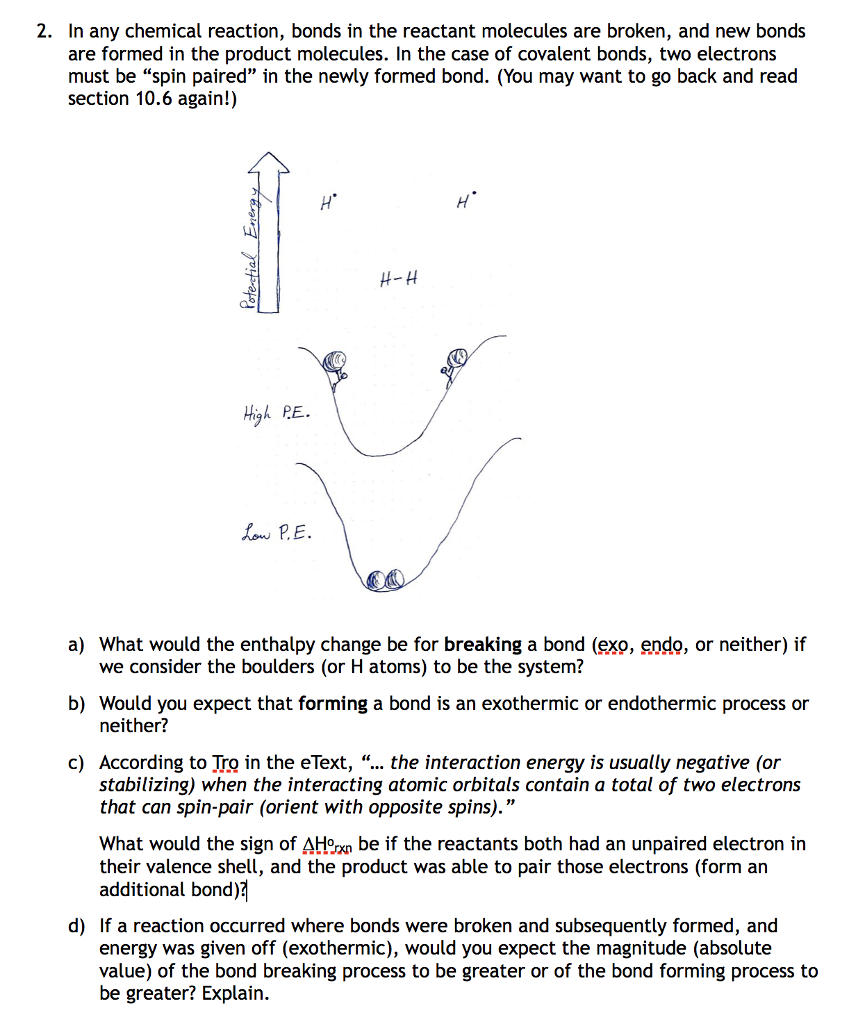
Solved 2. In any chemical reaction, bonds in the reactant
Web what is required for reactants to form bonds? Web the reactants must have the correct chemical properties to allow for bond formation. As a result, the surroundings get cold. Web bond making is generally an endothermic reaction so input energy would be required to make a reactants bond. This means that the atoms or molecules must be positioned in.
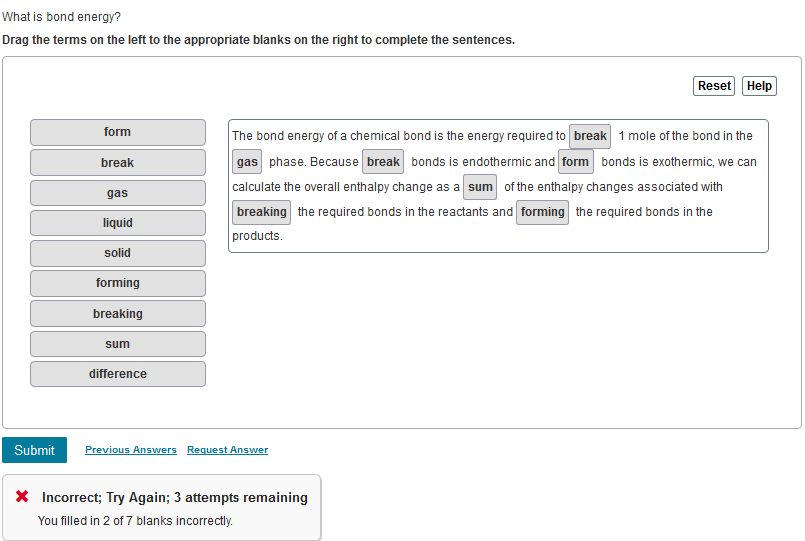
Solved What is bond energy? Drag the terms on the left to
Web in the cytoplasm, ribsomal rna (rrna), a type of rna, and protein combine to form a nucleoprotein called a ribosome. Web what is required for reactants to form bonds? Web bond making is generally an endothermic reaction so input energy would be required to make a reactants bond. The reactant molecules must collide. Whether a reaction is endothermic or.
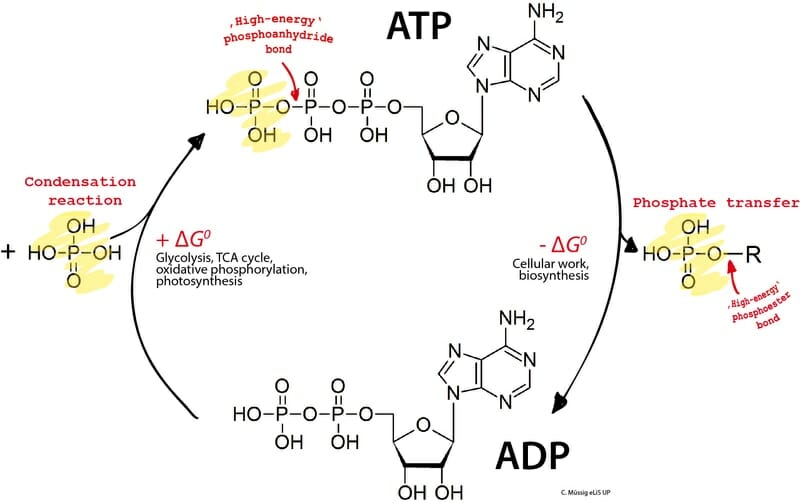
Dehydration Synthesis Definition and Examples Biology Dictionary
Energy is required to break bonds. Web answer answered what is required for reactants to form bonds? To start a reaction, energy must be absorbed by the chemicals in order to break some of the chemical bondsin the reactants. Web heat is absorbed by reactants to form products. Web what is required for reactants to form bonds?
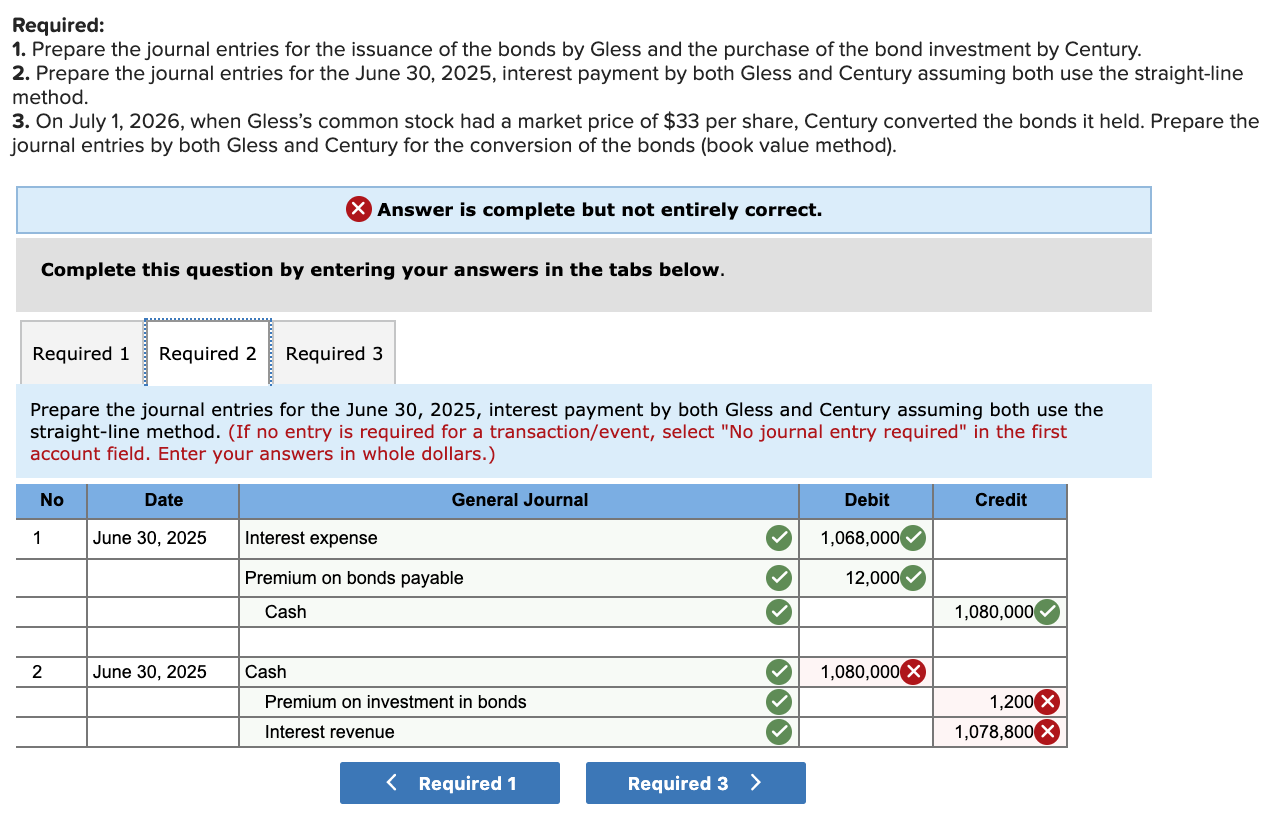
Solved Exercise 1424 (Algo) Convertible bonds;
Energy is required to break bonds. Web activation energy is required to weaken the bonds of reactants or toconvert the reactants into activated complex which leads to the products. Web what is required for reactants to form bonds? A compound partitions into its components. As a result, the surroundings get cold.
Bond Energy Calculations & Enthalpy Change Problems, Basic Introduction
Web answer answered what is required for reactants to form bonds? A compound partitions into its components. Energy output energy input equilibrium exchange reaction answers answer 1 energy input is required for reactants to form. Web in order for reactants to form bonds, three conditions need to be satisfied: As a result, the surroundings get cold.
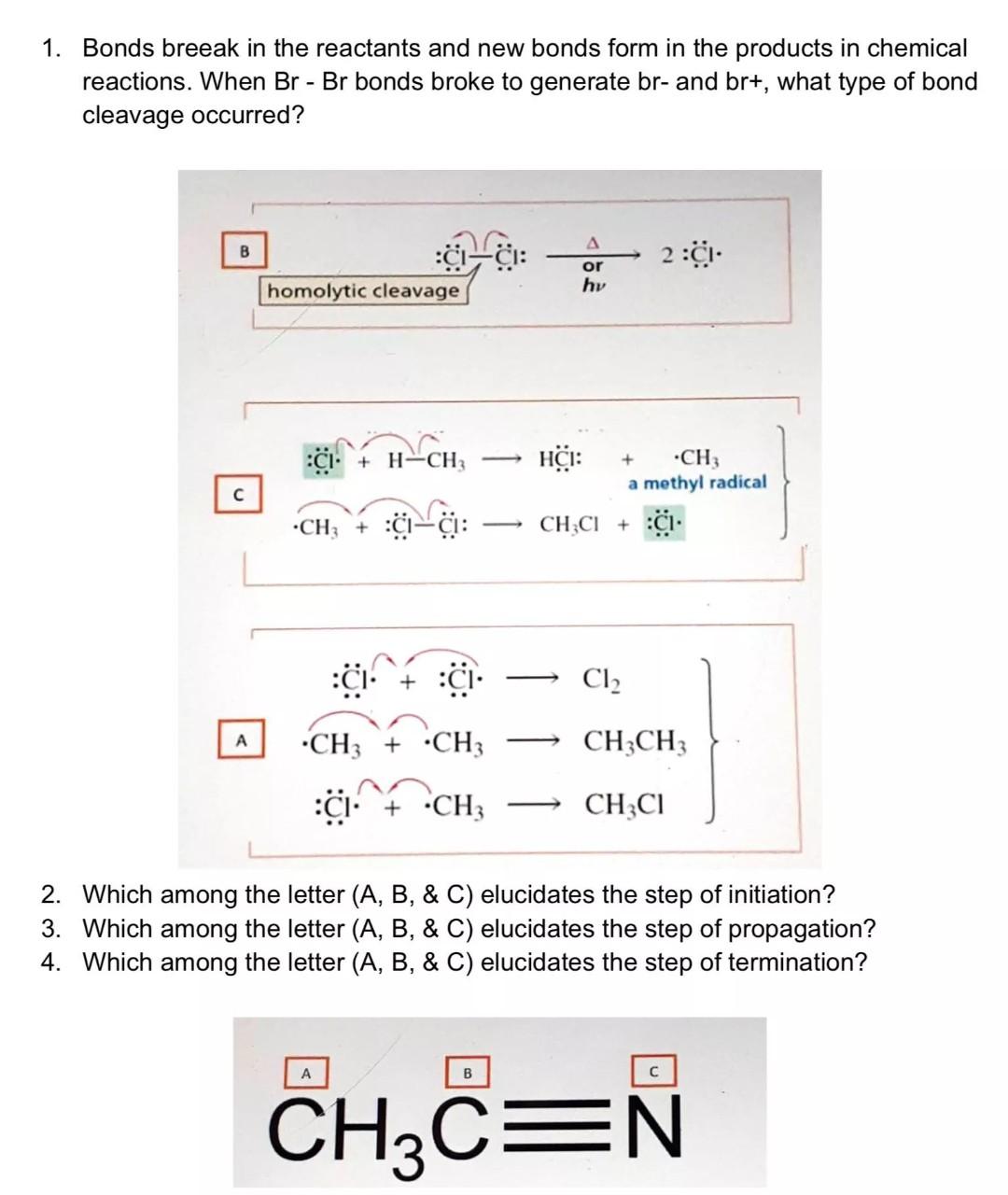
Solved 1. Bonds breeak in the reactants and new bonds form
The ribosome serves as the site and carries the. This means that the atoms or molecules must be positioned in a way that allows for the. Whether a reaction is endothermic or exothermic depends on the difference between the. Web atoms are held together by a certain amount of energy called bond energy. Web bond making is generally an endothermic.
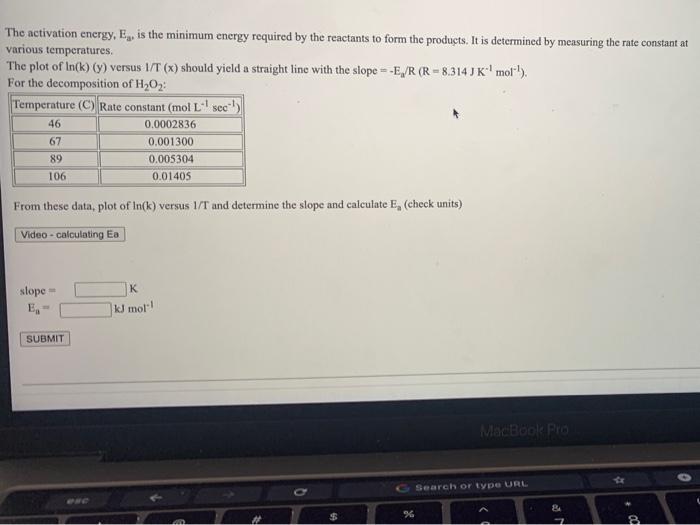
The activation energy, E., is the minimum energy
Web energy is released when new bonds form. Web bond making is generally an endothermic reaction so input energy would be required to make a reactants bond. See answer advertisement beckah initial energy input that meets or exceeds the activation energy (the amount of. This means that the atoms or molecules must be positioned in a way that allows for.
Premium Bonds Application Form Premium Bonds Application Form Pdf
Web in order for reactants to form bonds, three conditions need to be satisfied: Web atoms are held together by a certain amount of energy called bond energy. Energy output energy input equilibrium exchange reaction answers answer 1 energy input is required for reactants to form. A compound partitions into its components. Web what is required for reactants to form.
Solved The Elements In Group 2A Are Known By What Name? C...
For example, the bonds of two water. The reactant molecules must collide. Web enzyme the reactants used to form the products can also be synthesized from those products reversible reaction that which is produced as a result of a chemical reaction. The amount of energy needed to. Web bond making is generally an endothermic reaction so input energy would be.
See Answer Advertisement Beckah Initial Energy Input That Meets Or Exceeds The Activation Energy (The Amount Of.
Web atoms are held together by a certain amount of energy called bond energy. Web what is required for reactants to form bonds? Heat is absorbed from the surroundings; Web what is required for reactants to form bonds?
Web Enzyme The Reactants Used To Form The Products Can Also Be Synthesized From Those Products Reversible Reaction That Which Is Produced As A Result Of A Chemical Reaction.
The reactants must have the correct orientation and geometry to allow for bonding. As a result, the surroundings get cold. Energy is required to break bonds. Web when a chemical reaction occurs, molecular bonds are broken and other bonds are formed to make different molecules.
Web In The Cytoplasm, Ribsomal Rna (Rrna), A Type Of Rna, And Protein Combine To Form A Nucleoprotein Called A Ribosome.
Web answer answered what is required for reactants to form bonds? The reactant molecules must collide. Web what is required for reactants to form bonds? For example, the bonds of two water.
A Compound Partitions Into Its Components.
The amount of energy needed to. This includes having the right number and type of valence electrons, as well as the ability to. Web heat is absorbed by reactants to form products. Web energy is released when new bonds form.