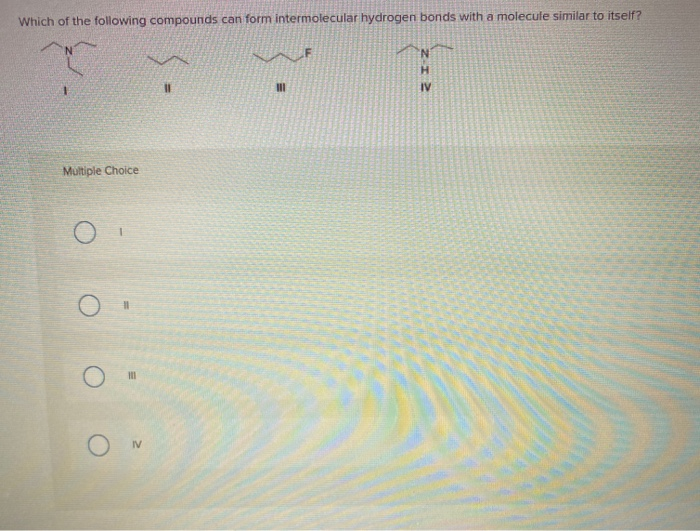
Which Of The Following Can Form Intermolecular Hydrogen Bonds
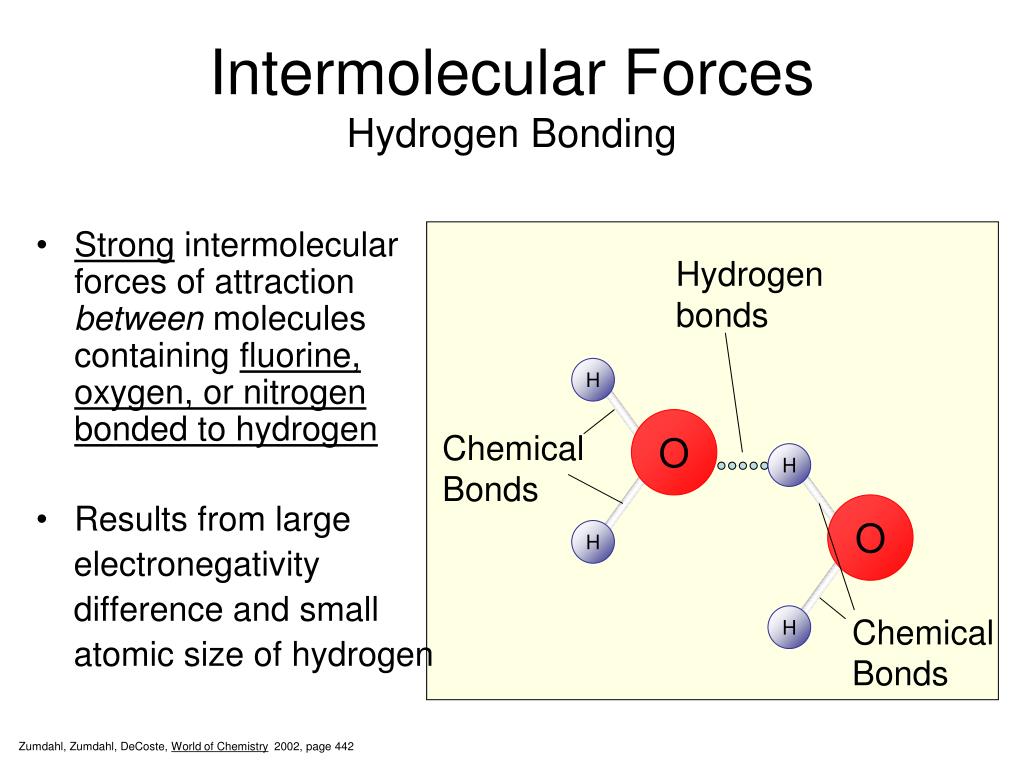
Which Of The Following Can Form Intermolecular Hydrogen Bonds - Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a partially positive hydrogen atom that is covalently bound to a different n or o atom. Hydrogen bonds have strengths ranging from 5 kj/mol to 50 kj/mol. Those bonds are represented between hydrogen and ah hah login, which is x, the halogen. E) all of these compounds can form hydrogen bonds. In summary, hydrogen bonds are. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web this type of intermolecular bond is stronger than london dispersion forces with the same number of electrons. Web intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °c) compared to the other group 16 hydrides, which have little capability to hydrogen bond. Some compounds can form into molecular hydrogen bonds. What types of intermolecular forces exist in a sample of acetone?
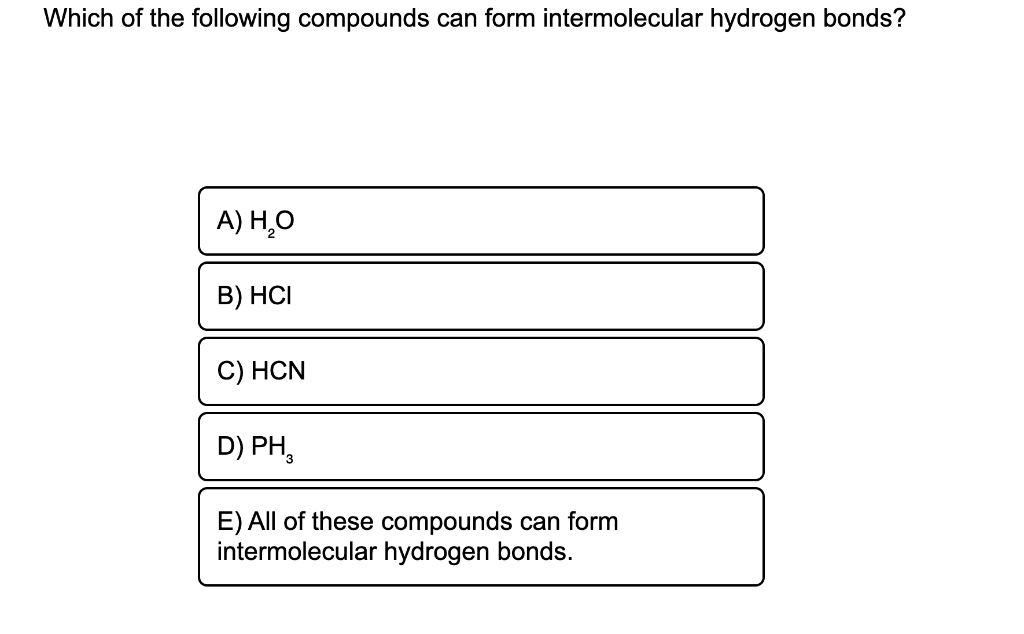
Web this type of intermolecular bond is stronger than london dispersion forces with the same number of electrons. Some compounds can form into molecular hydrogen bonds. A) h2o b) hcl c) hcn d) ph3 e) all of these can form intermoleculat hydrogen bonds Which of the following compounds can form intermolecular hydrogen bonds?. Web class 11 >> chemistry >> chemical bonding and molecular structure >> hydrogen bonding >> which of the following compounds can for question which of the. Hydrogen bonds have strengths ranging from 5 kj/mol to 50 kj/mol. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. Web hydrogen bonding in organic molecules containing nitrogen. Web which of the following compounds can form intermolecular hydrogen bonds?
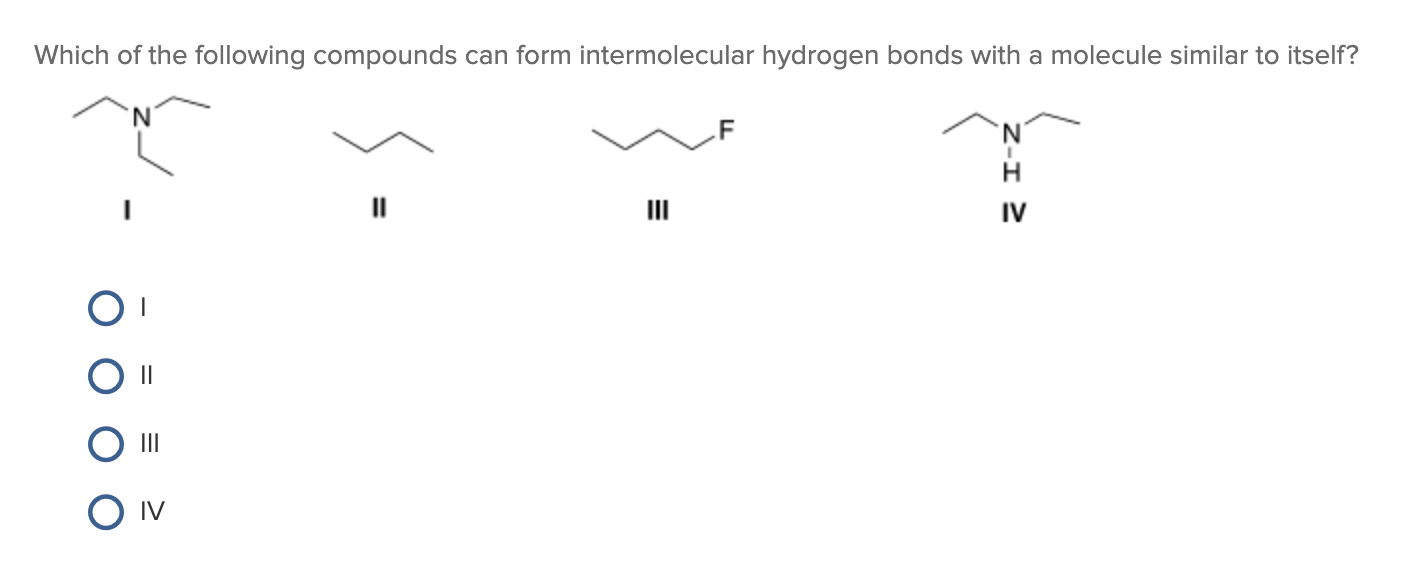
Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. Which of the following compounds can form intermolecular hydrogen bonds?. Web which of the following compounds can form intermolecular hydrogen bonds? A) h2o b) hcl c) hcn d) ph3 e) all of these can form intermoleculat hydrogen bonds Those bonds are represented between hydrogen and ah hah login, which is x, the halogen. Web this type of intermolecular bond is stronger than london dispersion forces with the same number of electrons. What types of intermolecular forces exist in a sample of acetone? Web hydrogen bonding in organic molecules containing nitrogen. Hydrogen bonding is the strongest type of. (select all that apply.) hf br2 ch3oh ch4
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a partially positive hydrogen atom that is covalently bound to a different n or o atom. E) all of these compounds can form hydrogen bonds. Web this type of intermolecular bond is stronger than london dispersion forces with the same number of electrons..
Image result for intermolecular forces
In summary, hydrogen bonds are. Hydrogen bonding is the strongest type of. Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a partially positive hydrogen atom that is covalently bound to a different n or o atom. A) h2o b) hcl c) hcn d) ph3 e) all of these can form intermoleculat.
PPT Intermolecular Forces PowerPoint Presentation ID705859
A) h2o b) hcl c) hcn d) ph3 e) all of these can form intermoleculat hydrogen bonds Web which of the following compounds can form intermolecular hydrogen bonds? Hydrogen bonds have strengths ranging from 5 kj/mol to 50 kj/mol. Which of the following compounds can form intermolecular hydrogen bonds? Which of the following compounds can form intermolecular hydrogen bonds?.
Solved Which of the following compounds can form
Web class 11 >> chemistry >> chemical bonding and molecular structure >> hydrogen bonding >> which of the following compounds can for question which of the. Which of the following compounds can form intermolecular hydrogen bonds? Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? Web intermolecular hydrogen bonding is responsible for the high.
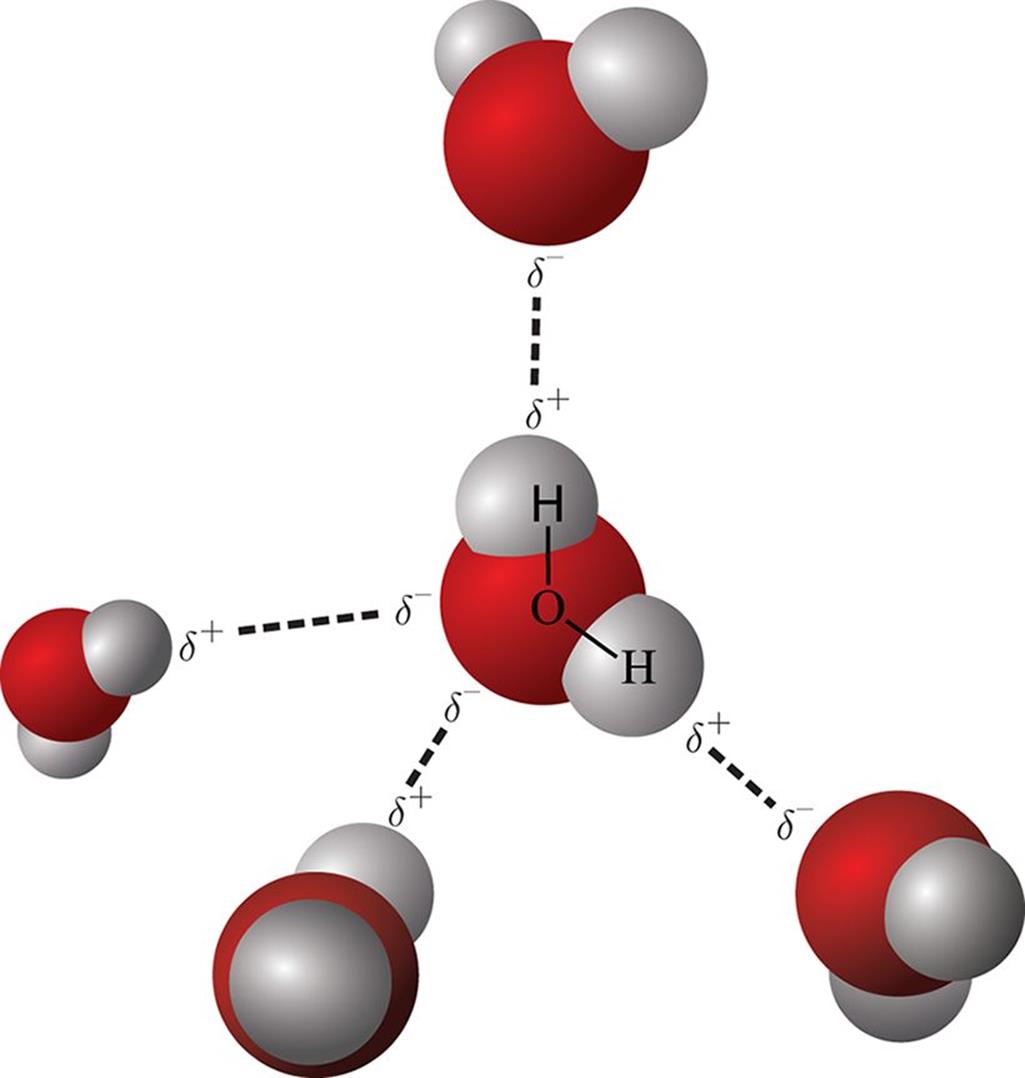
Figure 3.15. Hydrogen Bonding in Water
A) h2o b) hcl c) hcn d) ph3 e) all of these can form intermoleculat hydrogen bonds Web class 11 >> chemistry >> chemical bonding and molecular structure >> hydrogen bonding >> which of the following compounds can for question which of the. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts..
Hydrogen Bonding in Ethanol (C2H5OH) YouTube
Some compounds can form into molecular hydrogen bonds. E) all of these compounds can form hydrogen bonds. In summary, hydrogen bonds are. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Which of the following compounds can form intermolecular hydrogen bonds?
IGCSE Physical and Chemical Properties of Hydrocarbons IGCSE And IAL
Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? E) all of these compounds can form hydrogen bonds. Web class 11 >> chemistry >> chemical bonding and molecular structure >> hydrogen bonding >> which of the following compounds can for question which of the. Hydrogen bonds have strengths ranging from 5 kj/mol to 50.
Solved Which of the following compounds can form
What types of intermolecular forces exist in a sample of acetone? Hydrogen bonds have strengths ranging from 5 kj/mol to 50 kj/mol. Which of the following compounds can form intermolecular hydrogen bonds? Web intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °c) compared to the other group 16 hydrides, which have little capability to hydrogen.
Diagram Of Water Molecules Hydrogen Bonding
Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? Web hydrogen bonding in organic molecules containing nitrogen. Which of the following compounds can form intermolecular hydrogen bonds? E) all of these compounds can form hydrogen bonds. Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a.
Solved Which Of The Following Compounds Can Form Intermol...
Web hydrogen bonding in organic molecules containing nitrogen. Those bonds are represented between hydrogen and ah hah login, which is x, the halogen. Some compounds can form into molecular hydrogen bonds. A) h2o b) hcl c) hcn d) ph3 e) all of these can form intermoleculat hydrogen bonds Web which of the following compounds will form intermolecular hydrogen bonds in.
Those Bonds Are Represented Between Hydrogen And Ah Hah Login, Which Is X, The Halogen.
Which of the following compounds can form intermolecular hydrogen bonds?. What types of intermolecular forces exist in a sample of acetone? Web hydrogen bonding in organic molecules containing nitrogen. Web which of the following compounds can form intermolecular hydrogen bonds?
Hydrogen Bonds Have Strengths Ranging From 5 Kj/Mol To 50 Kj/Mol.
Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. E) all of these compounds can form hydrogen bonds. Web intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °c) compared to the other group 16 hydrides, which have little capability to hydrogen bond. Web this type of intermolecular bond is stronger than london dispersion forces with the same number of electrons.
Web You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Web class 11 >> chemistry >> chemical bonding and molecular structure >> hydrogen bonding >> which of the following compounds can for question which of the. Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? Web a hydrogen bond is an electrostatic attraction between a partially negative n or o atom and a partially positive hydrogen atom that is covalently bound to a different n or o atom. (select all that apply.) hf br2 ch3oh ch4
Some Compounds Can Form Into Molecular Hydrogen Bonds.
In summary, hydrogen bonds are. Which of the following compounds can form intermolecular hydrogen bonds? A) h2o b) hcl c) hcn d) ph3 e) all of these can form intermoleculat hydrogen bonds Hydrogen bonding is the strongest type of.