How Many Covalent Bonds Can Chlorine Form
How Many Covalent Bonds Can Chlorine Form - Web 4 rows the slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom,. Group 5a form 3 bonds; Web sodium has an atomic number of 11, hence, sodium has one electron in its outer electron shell. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. And group 7a form one bond. Web two nitrogen atoms can bond to form a triple covalent bond which will give each individual atom an additional 3 electrons for an octet. The more electrons that are shared between two. If it shares one of those with another chlorine atom (and the other one with the first), they can both. Web typically, the atoms of group 4a form 4 covalent bonds; 2cl2 + 2h2o → 4hcl +o2 (2).
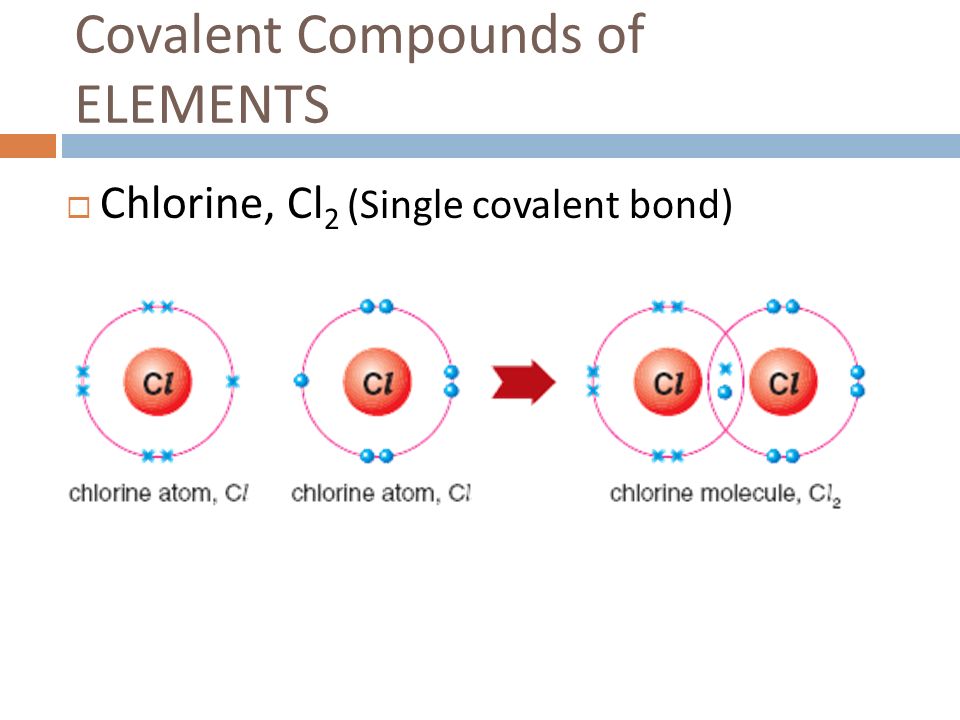
Web typically, the atoms of group 4a form 4 covalent bonds; Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; Chlorine, on the other hand, has an atomic number of 17 and has 7 electrons in its. Web the number refers to the number of bonds each of the element makes: Web in chlorine an electron pair is shared between the two atoms in cl 2. Web we refer to this as a pure covalent bond. Web two nitrogen atoms can bond to form a triple covalent bond which will give each individual atom an additional 3 electrons for an octet. They end of sharing 6 electrons between the. Group 6a form 2 bonds; Electrons shared in pure covalent bonds have an equal probability of being near each nucleus.
In the case of cl 2, each atom starts off. Web typically, the atoms of group 4a form 4 covalent bonds; They end of sharing 6 electrons between the. Web sodium has an atomic number of 11, hence, sodium has one electron in its outer electron shell. The more electrons that are shared between two. The halogens all have the general electron configuration ns2np5, giving them seven valence. Web usually each atom contributes one electron to the shared pair of electrons. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Web in chlorine an electron pair is shared between the two atoms in cl 2. So by sharing electrons through covalent bond formation, atoms are able to fill.
Solved How many covalent bonds can each element in the
Web typically, the atoms of group 4a form 4 covalent bonds; If it shares one of those with another chlorine atom (and the other one with the first), they can both. Web sodium has an atomic number of 11, hence, sodium has one electron in its outer electron shell. Group 5a form 3 bonds; Web one, two, or three pairs.
Covalent Bonding (Biology) — Definition & Role Expii
1 pm = 1 × 10 −12 m). Web sodium has an atomic number of 11, hence, sodium has one electron in its outer electron shell. Web typically, the atoms of group 4a form 4 covalent bonds; Web one, two, or three pairs of electrons may be shared between atoms, resulting in single, double, or triple bonds, respectively. This is.
Is SiO2 Ionic or Covalent? Techiescientist
Web cl2 +h2o → hocl + hcl (1) (1) c l 2 + h 2 o → h o c l + h c l at the boiling temperature of water, chlorine decomposes water: If it shares one of those with another chlorine atom (and the other one with the first), they can both. They end of sharing 6 electrons.
Bonding A Level Notes
Web cl2 +h2o → hocl + hcl (1) (1) c l 2 + h 2 o → h o c l + h c l at the boiling temperature of water, chlorine decomposes water: 2cl2 + 2h2o → 4hcl +o2 (2). Web typically, the atoms of group 4a form 4 covalent bonds; Web two nitrogen atoms can bond to form.
Ionic Bonds BOOKSTRONAUTS
Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Web cl2 +h2o → hocl + hcl (1) (1) c l 2 + h 2 o → h o c l + h c l at the boiling temperature of water, chlorine decomposes water: Chlorine, on the other hand, has an atomic number of 17.
__TOP__ How Many Covalent Bonds Can Chlorine Form
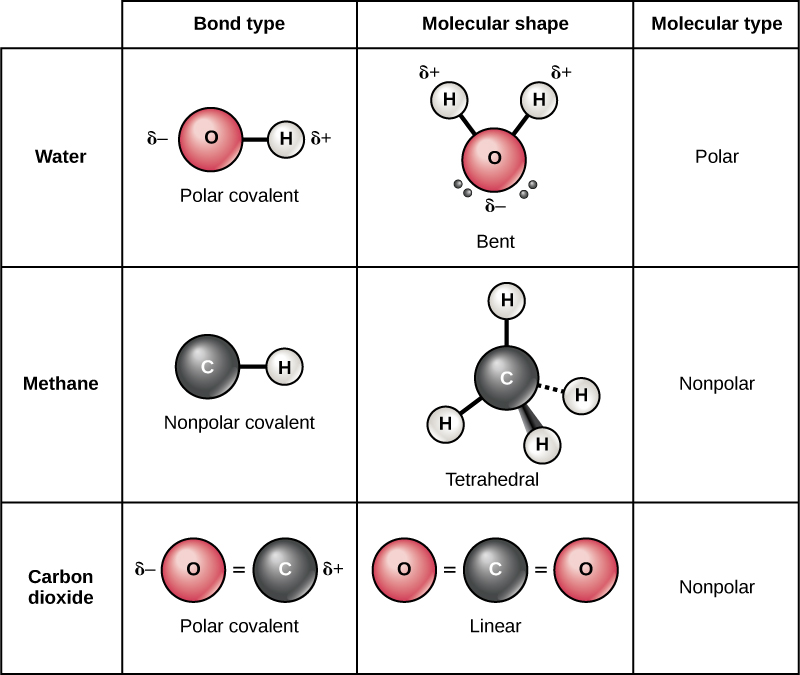
This is called covalent bonding. The more electrons that are shared between two. Web we refer to this as a pure covalent bond. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. They end of sharing 6 electrons between the.
How Many Covalent Bonds Can Calcium Form MaleahkruwJarvis
Group 6a form 2 bonds; Chlorine, on the other hand, has an atomic number of 17 and has 7 electrons in its. Web usually each atom contributes one electron to the shared pair of electrons. And group 7a form one bond. Group 5a form 3 bonds;
Chlorine combined with two negative atom or 1 positive and other
Chlorine, on the other hand, has an atomic number of 17 and has 7 electrons in its. They end of sharing 6 electrons between the. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; The halogens all have the general electron configuration ns2np5,.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Web in chlorine an electron pair is shared between the two atoms in cl 2. Web the number refers to the number of bonds each of the element makes: They end of sharing 6 electrons between the. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or.
Covalent Bond Biology Dictionary
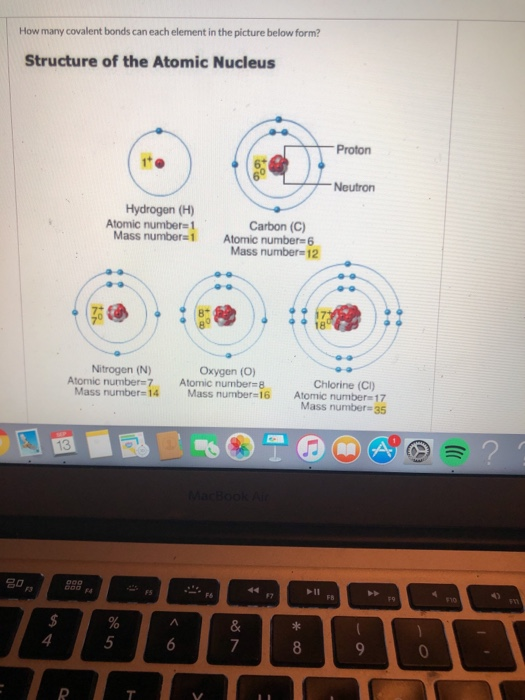
So by sharing electrons through covalent bond formation, atoms are able to fill. If it shares one of those with another chlorine atom (and the other one with the first), they can both. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. Web usually each atom contributes one electron to the shared.
Web Usually Each Atom Contributes One Electron To The Shared Pair Of Electrons.
Web how many covalent bonds can chlorine form? Group 6a form 2 bonds; Web in chlorine an electron pair is shared between the two atoms in cl 2. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus.
The More Electrons That Are Shared Between Two.
Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. The order of bonding, and so the valence state of cl in cloxxx−, x > 1 c l o x x x −, x > 1 compounds is very debatable. Chlorine, on the other hand, has an atomic number of 17 and has 7 electrons in its. Web 4 rows the slideshow shows a covalent bond being formed between a hydrogen atom and a chlorine atom,.
A Chlorine Atom Has 7 Electrons In Its Outer Shell.
And group 7a form one bond. Web cl2 +h2o → hocl + hcl (1) (1) c l 2 + h 2 o → h o c l + h c l at the boiling temperature of water, chlorine decomposes water: Web typically, the atoms of group 4a form 4 covalent bonds; 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy only one.
Web We Refer To This As A Pure Covalent Bond.
If it shares one of those with another chlorine atom (and the other one with the first), they can both. Web the number refers to the number of bonds each of the element makes: 1 pm = 1 × 10 −12 m). So by sharing electrons through covalent bond formation, atoms are able to fill.