Hydrochloric Acid And Sodium Hydroxide React To Form
Hydrochloric Acid And Sodium Hydroxide React To Form - It also explains how to predict the. Naoh(aq) + hcl(aq) rarr nacl(aq) + h_2o(l) because the sodium ions and the chloride ions are along for the. Web you can thus say that in this chemical reaction, two substances react sodium metal, na_ ((s)) hydrochloric acid, hcl_ ((aq)) and two substances are. Web answer verified 294.9k + views hint: Web answer (1 of 4): Web hydrochloric acid (hci) reacts with sodium hydroxide (naoh) to form sodium chloride (nacl) and water. Solid sodium hydroxide reacts with aqueous hydrochloric acid to form water and an aqueous solution of sodium chloride. Web solution the correct option is d sodium chloride hydrochloric acid is an acid while sodium hydroxide is a base. Web the balanced chemical equation is given by: As discussed previously, metals that are more active than acids.
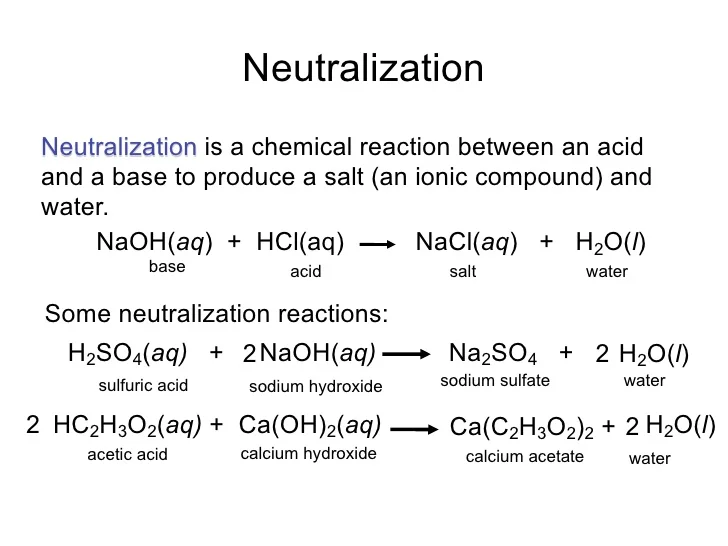
An acid reacts with a base to form salt and water. Web be sure your answer has the correct number of significant digits. Web when acids react with base they give salt and water and this reaction is known as neutralization reaction. Web the mass of sodium hydroxide will result in the same mass of sodium chloride. Hydrochloric acid (hcl) reacts with sodium hydroxide (naoh) to form sodium chloride (nacl) and water. Web the balanced chemical equation is given by: Web acids and bases react with metals. Web naoh ( aq) + hcl ( aq) → nacl ( aq) + h 2 o ( l) we know that sodium hydroxide ( naoh) is a base and hydrochloric acid ( hcl) is an acid. Web when hydrochloric acid and sodium hydroxide are mixed together? Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of.
As discussed previously, metals that are more active than acids. Web answer verified 294.9k + views hint: Web acids and bases react with metals. When strong acids such as hcl (hydrochloric acid) reacts with. Web you can thus say that in this chemical reaction, two substances react sodium metal, na_ ((s)) hydrochloric acid, hcl_ ((aq)) and two substances are. Web solution the correct option is d sodium chloride hydrochloric acid is an acid while sodium hydroxide is a base. It also explains how to predict the. Web hydrochloric acid (hci) reacts with sodium hydroxide (naoh) to form sodium chloride (nacl) and water. Web yes it is, it can be helpful to think of water as hydrogen hydroxide to see why this is true. Web the balanced chemical equation is given by:
neutralization hydrochloric acid sodium hydroxide chemistry
Web hydrochloric acid (hci) reacts with sodium hydroxide (naoh) to form sodium chloride (nacl) and water. Web when hydrochloric acid and sodium hydroxide are mixed together? It also explains how to predict the. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of. Web solution the correct option is d sodium chloride hydrochloric acid is an acid.
Sodium Hydroxide (NaoH) and Hydrochloric acid (HCL) reaction l Amazing
Web when hydrochloric acid and sodium hydroxide are mixed together? Web when acids react with base they give salt and water and this reaction is known as neutralization reaction. Naoh(aq) + hcl(aq) rarr nacl(aq) + h_2o(l) because the sodium ions and the chloride ions are along for the. Solid sodium hydroxide reacts with aqueous hydrochloric acid to form water and.
Hydrochloric Acid Sodium Hydroxide how to Make salt using
Web you can thus say that in this chemical reaction, two substances react sodium metal, na_ ((s)) hydrochloric acid, hcl_ ((aq)) and two substances are. Web solution the correct option is d sodium chloride hydrochloric acid is an acid while sodium hydroxide is a base. Web the mass of sodium hydroxide will result in the same mass of sodium chloride..
Sodium hydroxide reaction with hydrochloric acid
Web the mass of sodium hydroxide will result in the same mass of sodium chloride. Web the balanced chemical equation is given by: Web answer (1 of 4): Acids react with most metals to form a salt and hydrogen gas. The mass of hydrochloric acid will result in the same mass of sodium.
Sodium hydroxide and hydrochloric acid YouTube
Web this chemistry video tutorial explains how to write the net ionic equation between sodium hydroxide and hydrochloric acid. When strong acids such as hcl (hydrochloric acid) reacts with. Web naoh ( aq) + hcl ( aq) → nacl ( aq) + h 2 o ( l) we know that sodium hydroxide ( naoh) is a base and hydrochloric acid.
Sodium hydroxide, 0.1M, ampoule Vintessential Wine Laboratories
Web this chemistry video tutorial explains how to write the net ionic equation between sodium hydroxide and hydrochloric acid. An acid reacts with a base to form salt and water. Web solution the correct option is d sodium chloride hydrochloric acid is an acid while sodium hydroxide is a base. Web the balanced chemical equation is given by: Web acids.
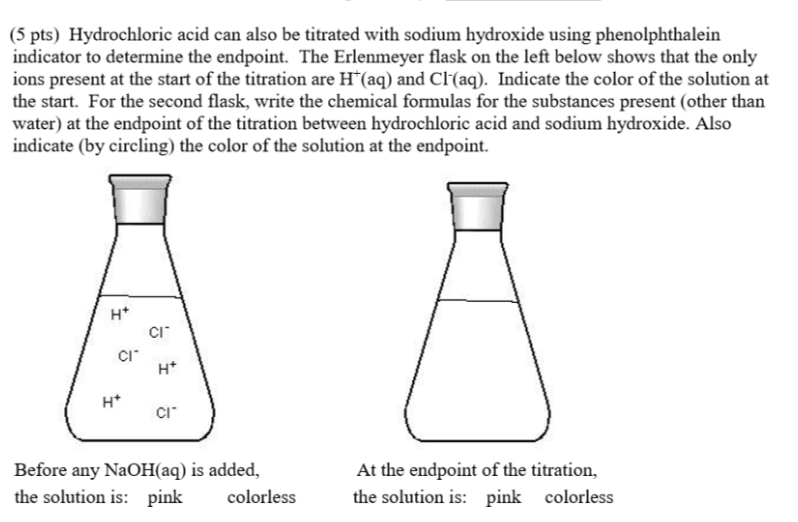
Solved Hydrochloric acid can also be titrated with sodium
Web be sure your answer has the correct number of significant digits. Web when acids react with base they give salt and water and this reaction is known as neutralization reaction. Hydrochloric acid (hcl) reacts with sodium hydroxide (naoh) to form sodium chloride (nacl) and water. It also explains how to predict the. Web answer (1 of 4):
🎉 Hydrochloric acid and sodium hydroxide. What is the equation for
Web naoh ( aq) + hcl ( aq) → nacl ( aq) + h 2 o ( l) we know that sodium hydroxide ( naoh) is a base and hydrochloric acid ( hcl) is an acid. Web be sure your answer has the correct number of significant digits. Web hydrochloric acid (hci) reacts with sodium hydroxide (naoh) to form sodium.
Sodium Hydroxide Reacts with Hydrochloric Acid Stock Image C036
Web when hydrochloric acid and sodium hydroxide are mixed together? As discussed previously, metals that are more active than acids. Web you can thus say that in this chemical reaction, two substances react sodium metal, na_ ((s)) hydrochloric acid, hcl_ ((aq)) and two substances are. Naoh(aq) + hcl(aq) rarr nacl(aq) + h_2o(l) because the sodium ions and the chloride ions.
FileSodiumHydroxide.jpg
Aqueous hydrochloric acid (hci) will react with solid sodium hydroxide (naoh) to produce. It also explains how to predict the. Web be sure your answer has the correct number of significant digits. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of. Web naoh ( aq) + hcl ( aq) → nacl ( aq) + h 2.
Web You Can Thus Say That In This Chemical Reaction, Two Substances React Sodium Metal, Na_ ((S)) Hydrochloric Acid, Hcl_ ((Aq)) And Two Substances Are.
Web when acids react with base they give salt and water and this reaction is known as neutralization reaction. It also explains how to predict the. Aqueous hydrochloric acid (hci) will react with solid sodium hydroxide (naoh) to produce. Web answer (1 of 4):
Web When Hydrochloric Acid And Sodium Hydroxide Are Mixed Together?
Web this chemistry video tutorial explains how to write the net ionic equation between sodium hydroxide and hydrochloric acid. Web naoh ( aq) + hcl ( aq) → nacl ( aq) + h 2 o ( l) we know that sodium hydroxide ( naoh) is a base and hydrochloric acid ( hcl) is an acid. Web hydrochloric acid (hci) reacts with sodium hydroxide (naoh) to form sodium chloride (nacl) and water. Naoh(aq) + hcl(aq) rarr nacl(aq) + h_2o(l) because the sodium ions and the chloride ions are along for the.
Hydrochloric Acid And Sodium Hydroxide Interact, Resulting In Salt And A Release Of.
Web be sure your answer has the correct number of significant digits. Web the mass of sodium hydroxide will result in the same mass of sodium chloride. Web the balanced chemical equation is given by: Web answer verified 294.9k + views hint:
Solid Sodium Hydroxide Reacts With Aqueous Hydrochloric Acid To Form Water And An Aqueous Solution Of Sodium Chloride.
As discussed previously, metals that are more active than acids. When strong acids such as hcl (hydrochloric acid) reacts with. The mass of hydrochloric acid will result in the same mass of sodium. Hydrochloric acid (hcl) reacts with sodium hydroxide (naoh) to form sodium chloride (nacl) and water.